ABSTRACT
Malignant pericardial effusion is common, being reported in 5–15% of all cancer patients. It most commonly arises from metastasis of lymphomas and of tumours of the lung, breast and, infrequently, the gastrointestinal tract. We describe the case of a 76-year-old woman who presented to the emergency room with cervical adenopathies and clinical signs of acute heart failure. The nodes were biopsied and found to be consistent with adenocarcinoma of the colon. CT showed thoracic lymphatic involvement but no evidence of other organ involvement. The patient developed cardiac tamponade and required emergent pericardiocentesis. To our knowledge, this is the first report of metastatic colon cancer without direct involvement of the pericardium or other solid organs.
LEARNING POINTS
- Large pericardial effusions are common in cancer patients.
- The pericardium in cancer patients may be affected by haematogenous or lymphatic spread or by local extension.
- A gastrointestinal origin of malignant pericardial effusions is rare but should be considered as a possible diagnosis.
KEYWORDS
Colon, cancer, metastasis, cardiac tamponade
INTRODUCTION
Malignant pericardial effusion occurs in some cancer patients[1] and commonly arises from metastatic disease[2]. Colon cancer is a common form of cancer[3] and 20% of patients already have metastatic disease at the time of diagnosis[3]. Colon cancer typically metastasizes to the regional lymph nodes, liver, lung and peritoneum[4].
Pericardial effusion is a common entity often overlooked in clinical practice. In developed countries, the aetiology remains undetermined in 50% of patients and is attributable to cancer in up to 25% of cases. In contrast, in developing countries, infections such as tuberculosis are the main causes of pericardial effusion (>60%)[5].
CASE PRESENTATION
We present the case of a 76-year-old woman with a history of hypercholesterolaemia, lupus and polyps of the colon. She was admitted to the emergency department with dyspnoea and orthopnoea for the previous month. She denied having fever, oedema or other signs and symptoms, particularly of the gastrointestinal tract. On examination the patient was oriented, afebrile and had normal vital signs. She had a distended jugular vein and audible crackles in the right lung base. A swollen lymph node approximately 1 cm in diameter was found in the internal portion of the left supraclavicular fossa, adherent to deeper tissues and painless on palpation. A second similar swollen lymph node also approximately 1 cm in diameter was found in the middle third of the right cervical ganglion chain. The rest of the examination was unremarkable, and the patient was admitted to the ward for further investigation.
Initial laboratory findings showed no changes in red or white blood cells, renal function was normal, and NT-proBNP was 254 pg/ml. An x-ray revealed bilateral pleural effusion, larger on the right side. A diagnostic pleural tap showed an exudate without neoplastic cells, and microbiological investigation, including mycobacteria, was negative.
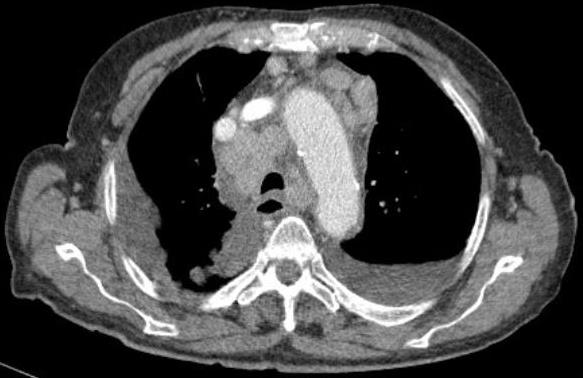
A CT of the neck, chest, abdomen and pelvis showed large bilateral adenopathies in the supraclavicular fossa, large mediastinal adenopathies (Fig. 1) and a large pericardial effusion as well as a bilateral pleural effusion (Fig. 2) without any other masses or suspicious nodes, and without involvement of the lung, liver or other solid organs.
Figure 1. Chest CT scan showing multiple adenopathies around the aortic arch and anteriorly to the heart
Figure 2. Chest CT scan showing A large pericardial effusion around the heart and bilateral pleural effusion
Histology of a biopsy specimen of the cervical adenopathy showed an adenocarcinoma of the colon with immunochemistry positive for CK20 and negative for CK7 and TTF-1. Bacterial examination of the adenopathy was negative as was culture for mycobacteria.
During her stay on the ward, the patient developed clinical signs of cardiac tamponade with dyspnoea, jugular distension and atrial flutter. She was transferred to the cardiology department where she underwent initial pericardiocentesis with drainage of 200 ml of haematic fluid. Despite this, the patient maintained a large symptomatic effusion, so a pericardial drain was placed with a total output of 725 ml of haematic fluid and which was removed on the fourth day. Cytology of the liquid was negative for neoplastic cells and the fluid was also negative for bacteria. A 1×0.5 cm biopsy specimen taken from the pericardium showed chronic pericarditis with inflammatory activity.
The patient eventually had a colonoscopy which showed a sessile polyp in the splenic angle. The biopsy showed a moderately differentiated adenocarcinoma. The patient was referred to the oncology department where palliative chemotherapy was initiated. Initially there was a very good response to chemotherapy and the patient lived for 2 more years with an excellent performance status but then died from her underlying cancer.
DISCUSSION
Colorectal cancer is the third most common cancer worldwide[3]. The portal system drains venous blood from the colon and proximal parts of the rectum to the liver and from there to the lungs via the heart. On the other hand, venous blood from the distal part of the rectum bypasses the liver and drains directly to the lung[4]. Autopsies of patients with cancer found that the pericardium was involved in 5–15% of cases[1].
It is thought that the pericardium in cancer patients may be affected via three possible pathways: haematogenous spread, lymphatic spread and local extension. Neoplastic pericardial involvement can lead to effusion through different mechanisms: obstruction of lymphatic and venous flow that increases hydrostatic pressure; overproduction of pericardial fluid due to exudation or direct leakage from tumour implantation in the pericardial leaflets; or a decrease in the oncotic pressure, usually due to indirect effects such as malnutrition and consequent hypoalbuminemia as well as cachexia[6]. The pattern of drainage in our patient was unusual as neither the liver nor the lungs were involved; only the lymphatic system was involved and was considered the main pathway of metastasis.
Signs in a patient with cardiac tamponade that make it more likely that it is associated with malignancy include the absence of two or more signs such as diffuse ST elevation, typical chest pain, pericardial rub and fever.
The presence of a pericardial rub is not as common in malignant pericardial effusions as in other aetiologies[6].
Although pericardial effusions are rare, large effusions are common in cancer patients. Primary tumours of the pericardium are infrequent, and most are secondary and result from metastasis from lung and breast cancer, malignant melanoma, lymphomas and leukaemia. Despite an increase in the frequency of this diagnosis in cancer patients, the majority will not have malignant invasion of the pericardium[2]. In our case the pericardial effusion seems to have resulted from lymphatic involvement of the mediastinum with impaired drainage. It should be kept in mind that cytology, which has a sensitivity of 67–92% for the detection of neoplastic cells, may produce a false negative result. A negative pericardial biopsy finding due to localized involvement of the pericardium can be confirmed through pericardioscopy in order to inspect both pericardial leaflets. However, since the pericardial effusion did not recur in our patient, it was decided not to perform a pericardioscopy. A negative biopsy is more likely when there is direct extension to the pericardium of the cancer, which was not the case in our patient[6].
CONCLUSION
The case discussed above is to the best of our knowledge the first described in the literature of cardiac tamponade as a manifestation of colon cancer without involvement of solid organs, namely the liver, or the pericardium. Although malignant pericardial effusions are common, the gastrointestinal origin of this condition is rare but should always be considered as a possible diagnosis[7].