ABSTRACT
Cardiac angiosarcoma (CA) is the most common primary malignant heart tumour. Its atypical symptoms and rapidly progressive nature contribute to delayed diagnosis and poor outcome. We report the case of a 52-year-old woman admitted with a large pericardial effusion. An extensive study of the aetiology of the pericardial effusion was inconclusive. Two months later the patient returned with ischaemic stroke. An echocardiogram revealed a probable right atrium contained rupture. The patient was submitted to surgical correction but died 9 days later. Histology revealed an angiosarcoma. This case exemplifies the atypical presentation of CA and highlights the importance of a multimodal diagnostic work-up in patients with idiopathic pericardial effusion.
LEARNING POINTS
- Cardiac angiosarcoma is often overlooked as an initial diagnosis because of its rarity and atypical symptoms, which, in association with its aggressiveness, contribute to delayed diagnosis and fatal outcome.
- Pericardial biopsy is an important technique that may help to disclose the aetiology of pericardial effusion and should be considered for the confirmation of malignant pericardial disease
- Patients presenting with pericardial effusion with cardiac tamponade with an unclear cause after diagnostic work-up should be followed closely.
KEYWORDS
Cardiac angiosarcoma, pericardial effusion, cardiac tamponade, pericardial biopsy, ischaemic stroke
CASE DESCRIPTION
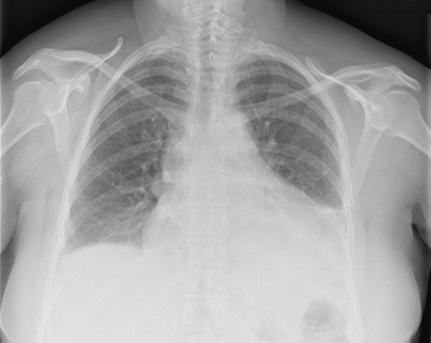
A 52-year-old Caucasian woman with an unremarkable medical history was admitted to the emergency department after one episode of syncope. She also complained of compressive precordial chest pain, with scapular irradiation, and exertional dyspnoea of 2 days’ duration. On physical examination, she was tachycardic (heart rate 111 bpm), with a blood pressure of 110/70 mmHg, a body temperature of 36ºC, and oxygen saturation of 94% on room air. There were crackles at both lung bases and diminished heart sounds. The remainder of the examination was normal. Laboratory tests revealed anaemia and elevated D-dimers. A chest x-ray showed an increase in the cardiac silhouette and left base hypotransparency (Fig. 1).
Figure 1. Chest x-ray showing an increase in the cardiac silhouette and left base hypotransparency suggestive of pleural effusion
Thoracic computed tomography (CT) angiography was performed and excluded an embolism but showed bilateral small pleural effusions and a large pericardial effusion. A transthoracic echocardiogram confirmed the pericardial effusion with the ‘swinging heart sign’ and substantial cardiac tamponade. Therefore, the patient underwent an emergent pericardiocentesis. Approximately 800 ml of bloody pericardial fluid was evacuated. The patient was then further investigated.
METHODS AND PROCEDURES
Pleural and pericardial fluid samples sent for study were found to be negative for bacteria, fungi, viruses and malignancy. Laboratory tests revealed iron-deficiency anaemia and an elevated erythrocyte sedimentation rate (107 mm/h). An autoimmune study (antinuclear antibodies, anti-double stranded DNA antibodies, rheumatoid factor) was negative, as was the infectious study, which included blood cultures and serological tests for Borrelia, Rickettsia conorii, Coxiella burnetii and Chlamydia pneumonia. To exclude an underlying neoplastic disease, a thoraco-abdominopelvic CT scan as well as gastrointestinal endoscopic studies were performed, with normal results. In addition to pericardiocentesis, the patient was treated with colchicine. She showed a good clinical outcome, with symptom resolution. The diagnosis of idiopathic pericardial effusion with cardiac tamponade at admission was assumed and the patient was discharged on colchicine. A month later she remained asymptomatic, with an unremarkable physical examination. However, 2 months later the patient presented with right central facial palsy and right arm paresis, with brain CT scanning showing a middle cerebral artery ischaemic stroke. A transoesophageal echocardiogram showed signs of a patent foramen ovale and a right atrium thrombus with a probable right atrium contained rupture (Fig. 2).
Figure 2. Transoesophageal echocardiogram showing the right atrium with a thrombus and signs of a contained rupture
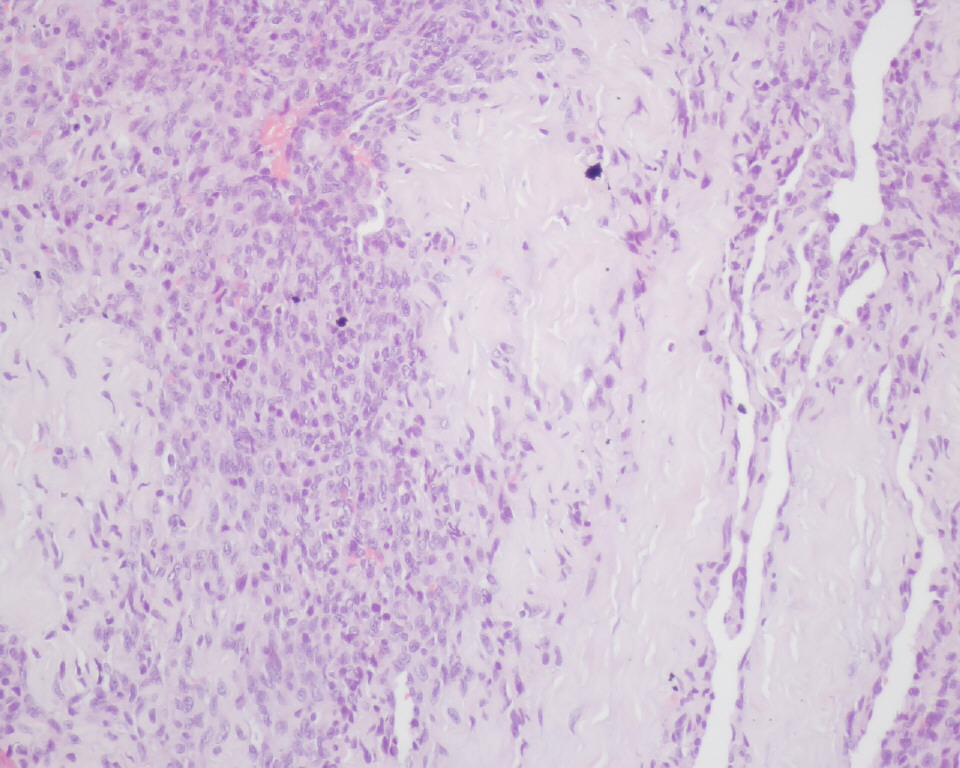
A thoracic CT scan revealed several pulmonary nodules suggesting metastasis. The patient was transferred to the cardiothoracic surgery department where she underwent surgical correction of the right atrium rupture. Unfortunately, on the 9th day after surgery she developed late cardiac tamponade and died. Right atrium and pericardium histological and immunohistochemical examination revealed polyhedral fusiform neoplastic cells, strongly positive for CD34 and CD31, and weakly positive for factor VIII (Fig. 3), consistent with cardiac angiosarcoma (CA).
Figure 3. Histology (haematoxylin–eosin at 200× magnification) of the right atrium showing replacement of the atrial wall with polyhedral fusiform neoplastic cells, consistent with cardiac angiosarcoma
DISCUSSION
Angiosarcoma is the most rapidly fatal primary malignant cardiac tumour and has usually already spread to the lungs, liver and brain by the time of presentation. CA appears more commonly in males, in the third to fifth decades of life, and usually arises in the right atrium. Its non-specific symptomatology and aggressiveness contribute to delayed diagnosis and poor outcome[1]. When feasible, surgical excision is the treatment of choice and has demonstrated survival improvement[2]. Radiation, chemotherapy and immunotherapy as adjuvants to surgery may occasionally improve survival[3], which remains poor, being 1 year or less[2].
In our case, pericardial effusion with cardiac tamponade was the first presentation of CA. As described in the literature, malignancy is the most common cause of large pericardial effusion and cardiac tamponade, with pericardiocentesis being the cornerstone of treatment for cardiac tamponade[4,5]. Pericardial fluid cytology for the aetiological investigation of pericardial effusion has a low diagnostic yield[1,6]. Pericardial or epicardial biopsy substantially improves the aetiological diagnosis[6] and is normally reserved for cases with recurrent cardiac tamponade or persistence without a defined aetiology, as well as in selected cases of suspected neoplastic or tuberculous processes[4]. According to the 2015 European Society of Cardiology guidelines for the Diagnosis and Management of Pericardial Diseases, interventional techniques are essential for their diagnostic work-up, including fluid sample collection, pericardial biopsy and pericardial drainage. The samples should be studied using molecular and histological/immunohistological diagnostic methods. Extended pericardial drainage is recommended for patients with suspected or definite neoplastic pericardial effusion in order to prevent effusion recurrence and provide intrapericardial therapy[5].
Although pericardium involvement is common in CA, secondary neoplasms in the pericardium are far more common than primary tumours[1]. Therefore, our initial approach was directed at excluding secondary neoplasms. Consequently, an extensive study was performed and was negative.
Since no definite pericardial effusion aetiology was found at the first assessment, the authors considered other diagnostic investigations. Echocardiography is an important diagnostic tool for cardiac tumour identification and characterization (location, shape, size, attachment, mobility). Transoesophageal echocardiography has 97% sensitivity for detecting cardiac masses and has a higher resolution than transthoracic echocardiography for differentiating between benign and malignant tumours. On the other hand, cardiac magnetic resonance imaging is better for soft tissue characterization and helps to distinguish between different myocardial abnormalities and between thrombi and tumours[1] Additionally, pericardial biopsy with histological/immunohistochemical evaluation of biopsy specimens improves the probability of making a definite aetiological diagnosis[6].
In the described case, all these investigations were considered and we believe that a pericardial biopsy would have been very useful. However, due to the patient’s poor collaboration and refusal, and considering the risks of such procedures, we decided on close follow-up. Although a more invasive diagnostic approach may have led to an early diagnosis, we believe it would not have changed the outcome, given the aggressive nature of this malignancy.
Another unexpected feature of our case was the ischaemic cardioembolic stroke, resulting from a peritumoural thrombus paradoxical embolism, due to a patent foramen ovale. In fact, embolic phenomena are a well-recognized manifestation of CA, although they usually occur in the pulmonary circulation given the common location of the tumour in the right atrium[1,2].
In this case, the diagnosis was made after death, with histological confirmation of atrial wall replacement by tumour cells and immunohistochemistry positive for endothelial cell immunomarkers (CD34, CD31, factor VIII), as previously reported[2,7].
Survival time from symptom onset was 2.5 months, less than the mean time described in the literature, which may have been due to the cardiac rupture.
In conclusion, we present a rare case of CA with pulmonary metastasis, presenting with pericardial effusion with cardiac tamponade and complicated by cardioembolic ischaemic stroke. Clinicians should be aware of these atypical features, so that the diagnosis is not overlooked. We also highlight the importance of techniques, such as pericardial biopsy, in pericardial effusion diagnostic work-up and management. The patient should be followed closely if the aetiology remains unclear.