ABSTRACT
Olmesartan-associated enteropathy is a rare cause of severe enteropathy that should be considered in the differential diagnosis of patients with unexplained chronic diarrhoea. It may be difficult to recognise because of its clinical and histologic similarities to other clinical entities. The authors present the case of a 72-year-old woman with a 6-month clinical history of non-bloody diarrhoea and weight loss. Discontinuation of olmesartan resulted in clinical and histologic recovery, and therefore, physicians need to be aware of olmesartan-associated enteropathy in order to avoid unnecessary testing. Although rare, it is considered an emerging and underdiagnosed enteropathy.
LEARNING POINTS
- Olmesartan-associated enteropathy is characterised by chronic diarrhoea (often severe) and weight loss that is unresponsive to a gluten-free diet.
- When a patient presents with unexplained chronic diarrhoea, a detailed medication review is needed. If duodenal biopsies reveal villous atrophy and coeliac disease is excluded, drug-induced enteropathy is likely.
- Clinical response and histologic improvement are expected after olmesartan is withdrawn.
KEYWORDS
Olmesartan, diarrhoea, enteropathy
CASE REPORT
A 72-year-old woman was admitted to the hospital with a 6-month clinical history of non-bloody diarrhoea (4 to 5 stools daily) and unintentional weight loss of 14 kg (6% of her original weight). In the last month she had experienced asthenia and episodic abdominal pain. A course of oral empirical antibiotics was used without clinical benefit. Previous peripheral arthritis (right elbow and wrist) and 2 episodes of uveitis with a trial of steroids (the last one 3 months previously) were reported.
She denied any other symptoms suggestive of local or systemic infections, recent travel, consumption of contaminated food or water, animal contact or changes in diet or medications within the past few months.
The patient had a previous history of arterial hypertension, dyslipidaemia and type 2 diabetes. She was treated with amlodipine, olmesartan plus hydrochlorothiazide 20/12.5 mg (taken for more than 2.5 years), acetylsalicylic acid, simvastatin and metformin.
On clinical examination, she had no palpable mass or organomegaly and there was no palpable lymphadenopathy.
Blood tests showed normochromic, normocytic anaemia (haemoglobin 11.4 g/dl, N: 12–15) and mild hypoalbuminaemia (3.2 g/dl, N: 3.5–5.0). The leucocyte count, coagulation, erythrocyte sedimentation rate, C-reactive protein, ionogram, hepatic and liver function, immunoglobulin levels and serum thyroid stimulating hormone were all normal. Stool examination, namely cultures and parasites, were found to be negative. Coeliac serology results were negative for anti-transglutaminase, anti-gliadin or anti-endomysium antibodies. Immunological assays were positive for anti-nuclear antibody (titre 1:1280; homogeneous pattern). Anti-histones, anti-ds-DNA, ENA screen, anti-neutrophil cytoplasmic antibodies and the HLA-B27 blood test were negative. Faecal calprotectin was 836 mg/kg (N: 0–50).
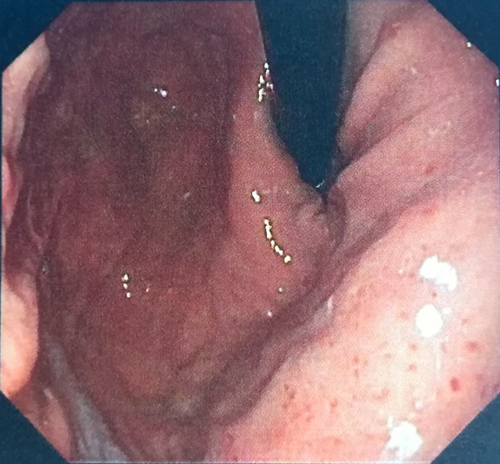
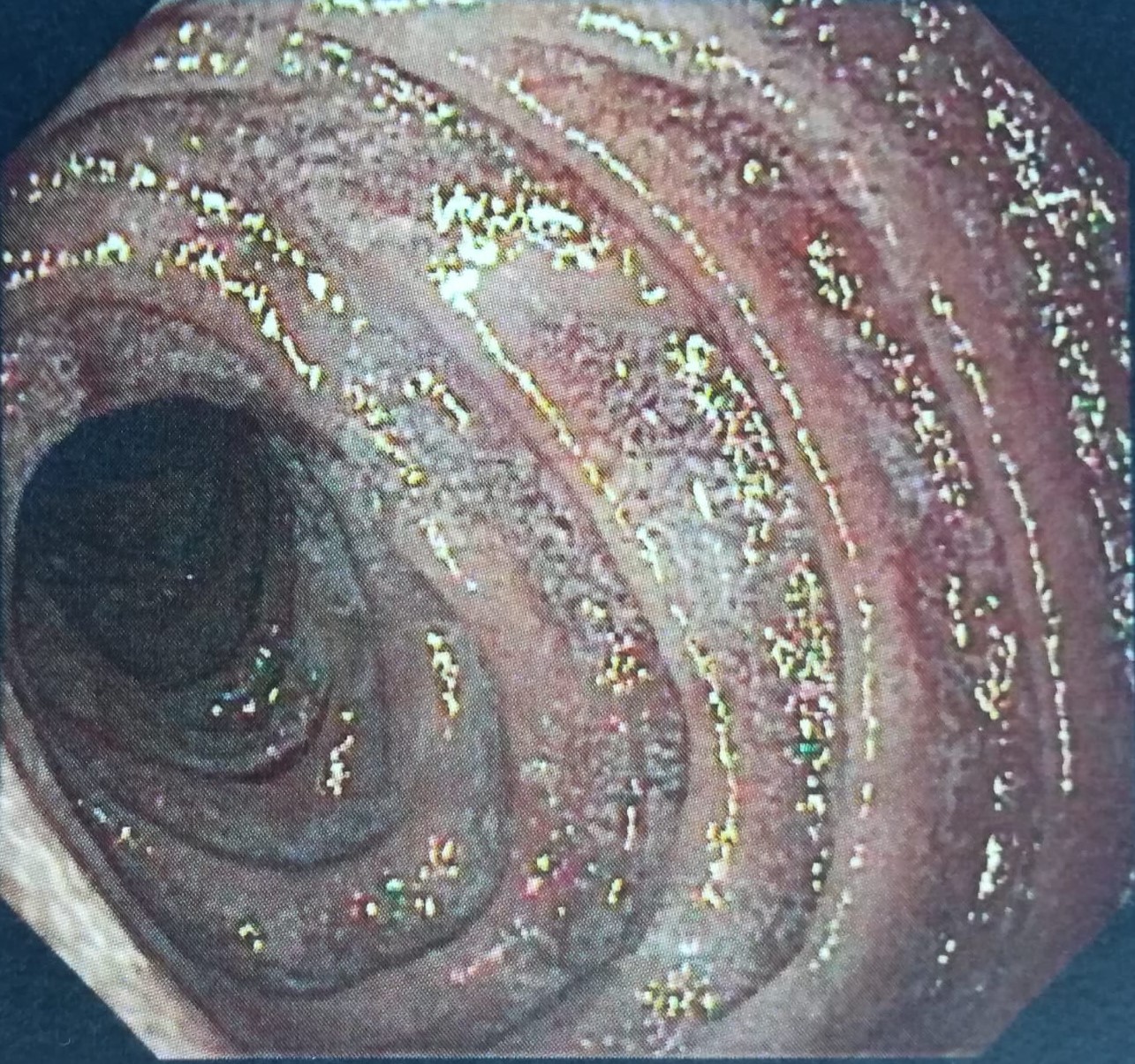
Thoraco–abdominal–pelvic CT showed normal results. Ileocolonoscopy was performed and there were no inflammatory or neoplastic changes. Colonic biopsies showed no evidence of microscopic colitis. Upper gastrointestinal endoscopy showed evidence of gastric hyperaemia (Helicobacter pylori gastritis) (Fig. 1) and mild attenuation of duodenal villous pattern (Fig. 2).Duodenal biopsy showed eosinophilic infiltration (73 eosinophils per high-power field), with no criteria of Whipple's disease.
Figure 1. Upper gastrointestinal endoscopy showed evidence of gastric hyperaemia.
Figure 2. Upper gastrointestinal endoscopy showed mild attenuation of the duodenal villous pattern.
The patient completed the treatment for Helicobacter pylori infection and once the possibility of olmesartan-associated enteropathy was considered she discontinued olmesartan. She has been asymptomatic since then, with clinical remission and weight gain. Reassessment at 4 months with upper endoscopy and biopsies showed histological recovery. We also verified at this time a decline in anti-nuclear antibody titre to 1:320. There is no evidence of additional musculoskeletal or ocular manifestations having occurred in the following 9 months.
DISCUSSION
Olmesartan is an angiotensin receptor blocker (ARB) commonly prescribed in the management of hypertension since 2002 in the USA, and from 2003 in the European Union. It acts by blocking the angiotensin II receptor and is usually well tolerated except for minor side effects, including dizziness and headache[1].
Enteropathy associated with olmesartan was first described by Rubio-Tapia et al in 2012 and is characterised by chronic diarrhoea (often severe) and weight loss that is unresponsive to a gluten-free diet[2]. Since then, additional cases and series have been reported with similar findings. If diarrhoea is a common adverse effect of medication, iatrogenic enteropathies with malabsorption syndrome are rarer.
The time between olmesartan exposure and onset of symptoms is highly variable and ranges from less than a few months to 5 years, with a mean duration of 3.1 years[1]. How olmesartan can induce severe inflammation and intestinal damage remains unknown. The long delay between the onset of olmesartan therapy and the development of enteropathy with diarrhoea is suggestive of a cell-mediated immune response[2].
Laboratory work-up commonly reveals non-specific anaemia, hypoalbuminaemia, electrolyte imbalance and vitamin deficiencies, consistent with a severe malabsorption process. Coeliac serology is always negative. The majority of patients may have either HLA-DQ2 or DQ8 haplotypes, suggesting increased risk of immune-mediated damage in these individuals[2].
Histopathological findings include a combination of duodenal villous atrophy (either total or partial), which may be associated with variable degrees of mucosal inflammation as granulocytic infiltration (both neutrophils and eosinophils), and a thickened subepithelial collagen layer. However, none of these biopsy features were statistically significant in terms of being a pathological marker of olmesartan-associated enteropathy. Reviews of the literature have shown that the spectrum of histologic changes is extensive and variable and may include the entire gastrointestinal tract[2,3].
In cases of villous atrophy with negative coeliac serology, olmesartan-associated enteropathy is most likely, but several other disorders are possible. Differential diagnosis includes other drug-related enteropathies, autoimmune enteropathy, tropical sprue, small-bowel bacterial overgrowth, hypogammaglobulinaemic sprue, giardiasis, Whipple's disease, collagenous sprue and unclassified sprue[4]
Confirmation of diagnosis requires clinical resolution of symptoms after olmesartan interruption. Mucosal recovery is also expected within 3–6 months of olmesartan withdrawal and a follow-up duodenal biopsy should be conducted. Some patients with severe symptoms, and recurrent hospitalisations due to dehydration or a slow and/or partial response to olmesartan withdrawal may experience some benefit from a short course of steroids such as budesonide[4].
Although there have been sporadic case reports of enteropathy possibly associated with irbesartan, losartan and valsartan, there is a clear predominance of published data relating to olmesartan[4].
The patient described in this report had an extensive work-up for her chronic diarrhoea. Previous history of arthritis and uveitis, a high titre of anti-nuclear antibodies and fecal calprotectin suggested inflammatory bowel disease or enteropathic arthropathies, although patients tend to be younger.
In the absence of peripheric eosinophilia and clinicopathological evidence of other causes, the hypothesis of olmesartan-induced enteropathy remains more probable[5]. The prompt relief of symptoms and improved histology when olmesartan was withdrawn is strong evidence for an aetiologic association.
Olmesartan-associated enteropathy can mimic other diseases, and it is crucial for clinicians to be aware of this to avoid extensive investigations and delay in the diagnosis, as symptoms can be severe and/or life-threatening. A clinical response should be expected within days of olmesartan suspension.