ABSTRACT
Denosumab is an antiresorptive agent widely used for treating osteoporosis. Atypical femur fractures, osteonecrosis of the jaw and hypocalcaemia are well-known possible adverse effects of this drug. We present, to our knowledge, the first case report in the English literature of clinically significant interstitial lung disease likely related to denosumab.
LEARNING POINTS
- Denosumab is a fully human monoclonal antibody which may rarely cause interstitial lung disease (ILD).
- The findings from this isolated case report of ILD in a patient taking denosumab do not prove causality.
- Nevertheless, we suggest that patient exposure to denosumab should be considered in a patient with ILD.
KEYWORDS
Interstitial lung disease, denosumab, osteoporosis
INTRODUCTION
Denosumab is a fully human monoclonal antibody to the receptor activator of nuclear factor-κB ligand (RANKL) that blocks its binding to RANK, inhibiting the development and activity of osteoclasts[1,2]. However, since RANKL and RANK are also expressed in the lung[3,4], there is theoretical concern that denosumab might have adverse pulmonary effects. We describe, as far as we know, the first case report of clinically significant interstitial lung disease (ILD) likely related to denosumab.
CASE DESCRIPTION
In March 2018, an 87-year-old non-smoking woman presented with a 1-month history of dry cough and worsening exertional dyspnoea. Her past medical history was significant for arterial hypertension, atrial fibrillation and postmenopausal osteoporosis. She had been taking nebivolol, digoxin, acenocoumarol and pantoprazole for many years along with subcutaneous denosumab since May 2016 (60 mg every 6 months, the third dose having been given 4 months before admission). Physical examination was remarkable only for cardiac arrhythmia and fine, high-pitched bibasilar inspiratory crackles.
METHODS AND PROCEDURES
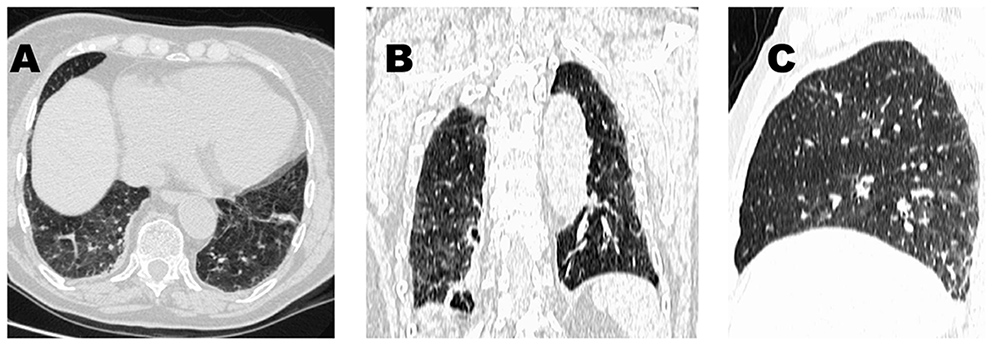
Oxygen therapy was prescribed since her arterial O2 partial pressure was 43 mmHg while breathing room air. The white blood cell count and differential, haemoglobin concentration and blood urea nitrogen were all normal; the ESR was 38 mm/hour. The chest radiograph revealed bilateral basal reticular abnormalities. An echocardiographic study was normal. A high-resolution CT scan of the chest displayed a pattern suggestive of hypersensitivity pneumonitis (Fig. 1).
Diagnostic work-up for antinuclear antibodies, antineutrophil cytoplasmic antibodies, rheumatoid factor and angiotensin-converting enzyme showed normal results. Serology for HIV was negative. ILD related to denosumab was suspected and so this drug was discontinued while the other habitual medications were maintained. The patient was started on prednisone (10 mg/day as initial dose) and discharged on the 7th hospital day with recommendations for zoledronic acid treatment. Six months after discharge (while on prednisone 5 mg/day), she denied having dyspnoea, ESR was normal, and a high-resolution CT scan of the chest displayed normal findings. At a follow-up visit in early December 2018, the patient remained well and prednisone was stopped. When last seen on 9 May 2019, she continued to be eupnoeic, her arterial oxygen saturation was 95% while breathing room air, lung auscultation revealed no abnormalities, and the serum level of C-reactive protein was within normal limits. Denosumab re-challenge was avoided because both the adverse reaction had been severe (grade 3: symptomatic, interfering with activities of daily living or oxygen indicated[5]) and denosumab was regarded as a non-essential medication.
Figure 1. Chest high-resolution CT images in a patient with interstitial lung disease while on denosumab: axial (A), coronal (B) and sagittal (C) reconstructions show mosaic attenuation and scattered ground-glass opacities as well as mild reticulation, ill-defined lobular micronodules, and minimal architectural distortion. Note that there is no honeycombing or traction bronchiectasis
DISCUSSION
Drug-induced ILD incidence rates vary between 4.1 and 12.4 cases/million/year and account for 3–5% of all prevalent ILD cases[5]. Over 350 drugs can cause drug-induced ILD, but this is often unrecognised until late in drug development or after launch[5]. Moreover, drug-induced ILD is a diagnosis of exclusion, which poses unique challenges for the treating physician. Cancer therapy agents (bleomycin, gemcitabine and others), followed by rheumatology drugs (methotrexate, leflunomide and others), amiodarone and antibiotics (nitrofurantoin, daptomycin) are the most common causes of drug-induced ILD[5].
ILD associated with denosumab seems to be an exceptional occurrence. In fact, neither the comprehensive Pneumotox website (www.pneumotox.com) nor a recent thorough review on drug-induced ILD[5] include denosumab as a causative agent of ILD. Moreover, a PubMed search using the search terms ‘denosumab’ and ‘interstitial lung disease’ revealed no previous case reports on this matter published in the English literature (accessed on 29 April 2019).
However, on the other hand, a study on ILD related to the administration of monoclonal antibodies which includes denosumab has been recently reported[6]. The aim of this analysis was to investigate the time-to-onset and onset pattern of drug-induced ILD after the administration of monoclonal antibodies through the use of the spontaneous adverse reaction reporting system of the Japanese Adverse Drug Event Report (JADER) database. In conclusion, the results of the study showed that with the exception of denosumab (64.5 days) and adalimumab (126 days), the time-to-onset of drug-induced ILD for monoclonal antibody agents ranged from 1 to 2 months after the initial administration. Nevertheless, it is worth stating here that, as the authors of this report pointed out, there are several problems associated with spontaneous reporting databases, such as the JADER, that need to be taken into account. These problems include: (a) the size and characteristics of the target population are often unclear, which indicates that the usefulness of the obtained results cannot be definitively established; (b) due to bias in the process of obtaining and transferring information, it is difficult to evaluate the magnitude of the potential adverse event; (c) there are countless confounding prognostic factors and covariates, thereby making it hard to investigate which of these might have truly influenced the results; (d) there is no way to confirm the causal relationship, even if a suspected side effect is found; and (e) there is difficulty ascertaining the accuracy of the report.
As a result, careful attention needs to be paid to any interpretation of the results from the JADER database.
In addition, a search on VigiBase (www.vigiaccess.org) resulted in 62 hits for denosumab and ILD (accessed on 29 April 2019). VigiBase, the WHO global database of individual case safety reports, is the largest and most comprehensive database in the world, and is developed and maintained by the Uppsala Monitoring Centre on behalf of the WHO. It consists of reports of adverse reactions to medicines and vaccines received from member countries since 1968. VigiBase is updated with incoming case reports on a continuous basis. Although data from VigiBase can be published, VigiBase should not be regarded as a publication itself.
While the evidence from this single report is circumstantial, the history of drug exposure, absence of another more probable cause (e.g. infection, radiation-induced lung injury, or progression of an underlying disease), and the favourable clinical and radiological outcome upon denosumab withdrawal along with corticosteroid therapy are all consistent with a likely causal relationship. Alveolar macrophages express RANK and following its binding to RANKL, extracellular matrix degradation can be induced[4]. We hypothesize that denosumab, through blocking the RANKL-RANK binding, could potentially reduce extracellular matrix degradation by macrophages and lead in this way to ILD.
Plausible risk factors for developing denosumab-induced ILD in our patient could have been old age[5] and the possible presence of an idiosyncratic susceptibility. Why took it the patient nearly 2 years to develop ILD after starting denosumab treatment is a matter of discussion.
CONCLUSION
Although the findings from this isolated case do not prove causality, we suggest that until further data to support or refute the association are available, patient exposure to denosumab should be queried when a diagnosis of ILD is being considered.