ABSTRACT
Background: The occurrence of a high-risk pulmonary embolism (PE) within 48 hours of a complicated pericardiocentesis to remove a haemorrhagic pericardial effusion, is an uncommon clinical challenge.
Case summary: The authors report the case of a 75-year-old woman who presented with signs of imminent cardiac tamponade due to recurring idiopathic pericardial effusion. The patient underwent pericardiocentesis that was complicated by the loss of 1.5 litres of blood. Within 48 hours, the patient had collapsed with clear signs of obstructive shock. This was a life-threating situation so alteplase was administered after cardiac tamponade and hypertensive pneumothorax had been excluded. CT chest angiography later confirmed bilateral PE. The patient achieved haemodynamic stability less than an hour after receiving the alteplase. However, due to the high risk of bleeding, the medical team suspended the thrombolysis protocol and switched to unfractionated heparin within the hour. The cause of the PE was not identified despite extensive study, but after 1 year of follow-up the patient remained asymptomatic.
Discussion: Despite the presence of a contraindication, the use of thrombolytic therapy in obstructive shock after exclusion of hypertensive pneumothorax can be life-saving, and low-dose thrombolytic therapy may be a valid option in such cases.
LEARNING POINTS
- A quick and systematic approach to a collapsed patient with signs of shock is mandatory; understanding the type of shock may help narrow the differential diagnosis and help in therapeutic decisions.
- After exclusion of cardiac tamponade and hypertensive pneumothorax, life-saving thrombolytic therapy can be administered in obstructive shock due to probable massive pulmonary embolism.
- Contraindications for thrombolytic therapy originated as exclusion criteria for clinical trials but should not prevent the use of this therapy in life-threatening situations.
KEYWORDS
Pulmonary embolism, alteplase, pericardiocentesis, pericardial effusion, thrombolytic therapy
INTRODUCTION
We describe a clinical challenge where a patient experienced a high-risk pulmonary embolism (PE) within 48 hours of a complex pericardiocentesis to remove a haemorrhagic pericardial effusion. Despite recent advances, PE is still an important cause of hospital morbidity and mortality. When treated, PE has a short-term mortality rate of 1% in low-risk patients, but this increases to 35–58% in patients with shock[1]. Thrombolysis is the treatment of choice for acute PE associated with shock in patients without a high risk of bleeding[2].
The contraindications for thrombolytic therapy include haemorrhagic stroke, known bleeding risk and non-compressible vascular puncture[3]. However, there are no absolute contraindications in the context of impending haemodynamic collapse[3].
CASE DESCRIPTION
A 75-year-old Caucasian woman presented to a large teaching hospital with a 1-month history of progressive dyspnoea. She had a history of pericardial effusion since 2011, which had been drained twice. A definitive diagnosis had not been established despite extensive evaluation.
On admission the patient had a blood pressure (BP) of 122/60 mmHg with a heart rate (HR) of 65 bpm. A transthoracic echocardiogram was performed, showing a severe pericardial effusion measuring 38 mm posterior and 23 mm anterior, with a mild diastolic collapse of the right ventricle.
The next day a fluoroscopy-guided pericardiocentesis was performed and 1,000 ml of lightly blood-stained pericardial fluid was removed. However, the procedure was complicated by aspiration of a large volume (1,500 ml) of haematic fluid from an unknown site.
After the procedure, the patient became hypotensive but responded to a fluid bolus and a low dose of norepinephrine (0.1 µg/kg/min). Serial haemograms were performed showing a nadir of 7.5 g/dl of haemoglobin, so the patient was transfused with 2 units of packed red blood cells. The echocardiogram after the pericardiocentesis showed normal bi-ventricular function with a small pericardial effusion.
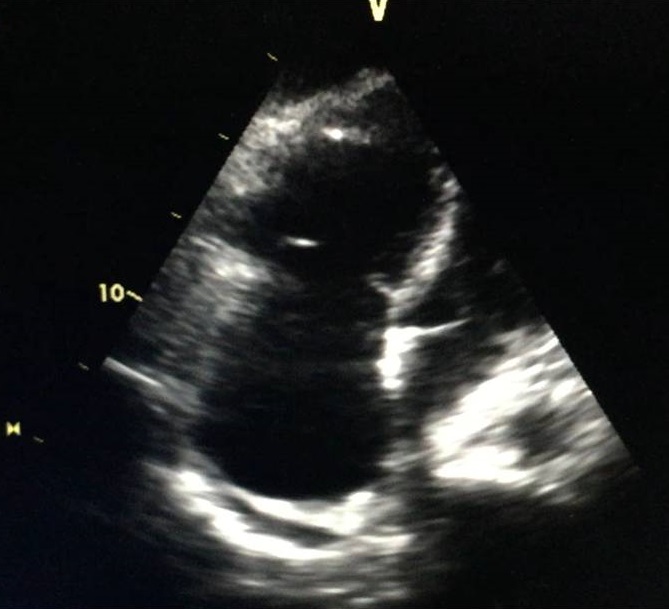
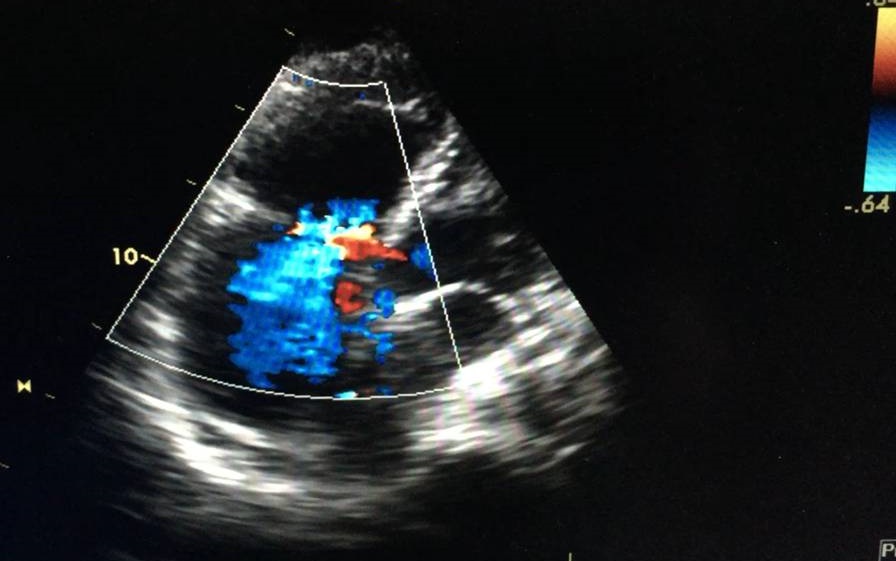
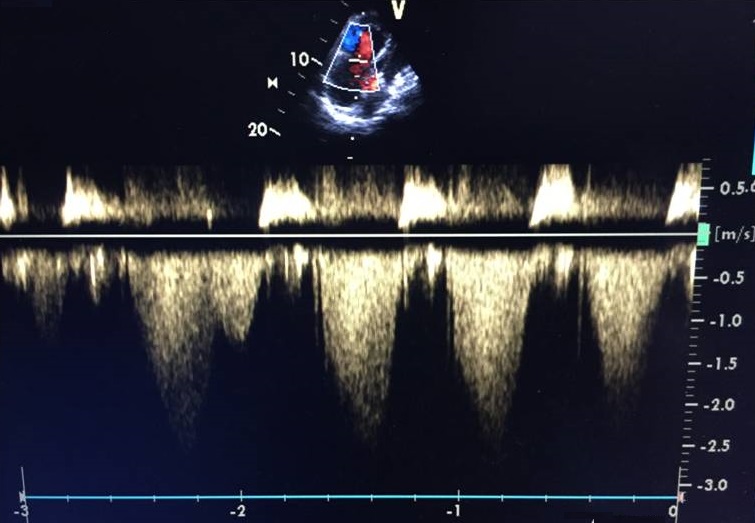
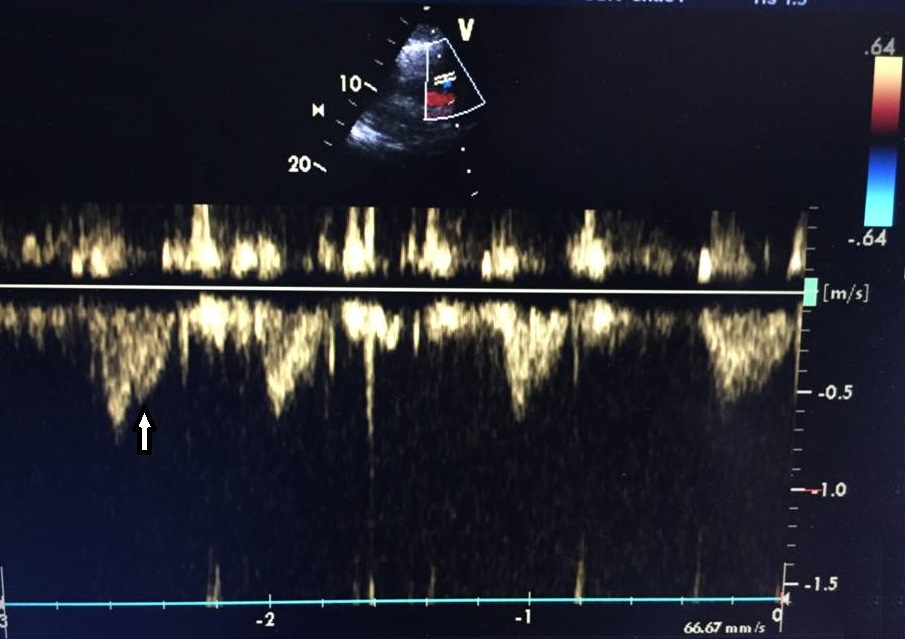
On the second day after the procedure, the patient was haemodynamically stable without norepinephrine so was allowed out of bed but then collapsed. Immediate examination showed BP of 84/40 mmHg and HR of 110 bpm. The patient was brought to the ICU, where she was agitated, restless and diaphoretic with a stabbing chest pain and cold extremities. Importantly, there was progressive worsening of her haemodynamic status and increasing chest pain. A fluid bolus was given and norepinephrine was reinitiated with no response. An echocardiogram was performed and excluded cardiac tamponade but showed a moderately to severely enlarged right ventricle (Fig. 1) with impaired systolic function and McConnell’s sign. Acute pulmonary hypertension was suggested by a mildly increased tricuspid regurgitation velocity and mid-systolic notching of the pulse wave Doppler profile in the right ventricular outflow tract (Figs. 2–4).
Figure 1. Transthoracic echocardiogram showing a moderately to severely enlarged right ventricle
Figure 2. Colour Doppler image showing severe tricuspid regurgitation
Figure 3. Peak tricuspid regurgitation with increased tricuspid regurgitation velocity
(-2.7 m/sec)
Figure 4. Rapid increase and mid-systolic notching
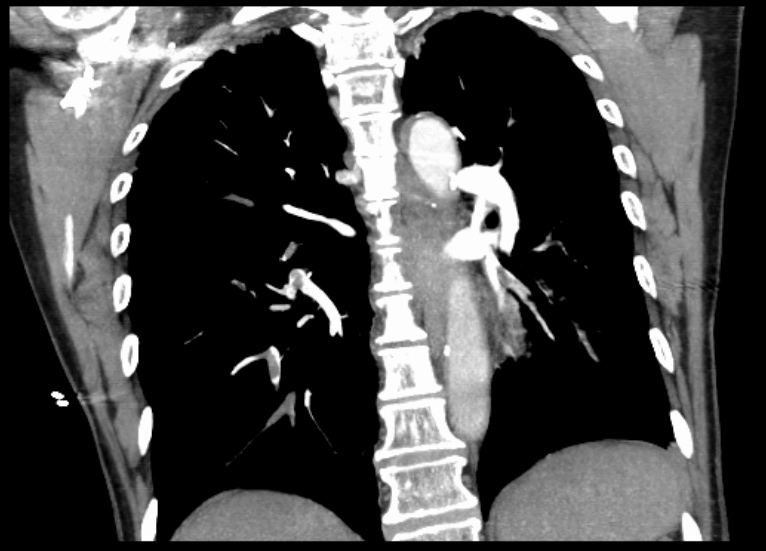
An emergency CT chest angiogram (CTA) was ordered, but the patient’s clinical situation deteriorated, so life-saving fibrinolysis was administered with a bolus of 10 mg of alteplase. Minutes after fibrinolysis, BP started to increase and the patient became less agitated. The CTA was eventually performed and showed signs of bilateral PE, involving the segmental branches of the right lung, left superior lobar branch and segmental branches of the lower left lobe (Fig. 5). After less than an hour the patient was haemodynamically stable and the chest pain had resolved.
Figure 5. CT chest angiogram showing bilateral pulmonary embolism, involving the segmental branches of the right lung, left superior lobar branch and segmental branches of the lower left lobea
Due to the very high bleeding risk and in light of the favourable initial response, the medical team did not follow the usual thrombolysis protocol (10 mg bolus followed by infusion of 90 mg of alteplase over 2 hours) but switched to unfractionated heparin (UFH) in the first hour. UFH was given for 24 hours, before being replaced with low molecular weight heparin. The patient was transferred to the general ward after 48 hours and discharged 5 days later. She was anticoagulated with apixaban 10 mg twice a day.
The aetiological study was repeated but no abnormalities were identified. Venous ultrasound of both limbs was negative for deep vein thrombosis. The patient was reviewed in the outpatient clinic for 1 year but was asymptomatic and echocardiograms were normal.
DISCUSSION
The collapse of a patient with signs of shock requires a fast and systematic review of the causes of the shock and appropriate therapy in order to reduce immediate mortality. In this case, the diagnosis of obstructive shock was quickly made and after exclusion of cardiac tamponade and hypertensive pneumothorax, the decision to treat probable massive PE with thrombolytic therapy was made despite the high risk of bleeding associated with the complicated pericardiocentesis performed less than 48 hours previously.
In this case, the authors decided to administer a bolus of alteplase according to the standard protocol, but after a good initial response and taking into account the high risk of bleeding, within an hour they switched to UFH infusion. As the major bleeding complications of alteplase are dose dependent and can affect up to 6.4% of patients, some trials have investigated whether low-dose alteplase has an adequate safety and efficacy profile, particularly in low-weight (<65 kg) patients and right ventricular dysfunction[4].
Most of the contraindications to thrombolytic therapy are based on expert consensus and originated as exclusion criteria in major stroke trials[5]. Recently, a review of thrombolytic therapy suggested that contraindications are unnecessarily restrictive in real-world clinical practice and pointed out that there is not an increased risk of bleeding in most contraindications. Additionally, there are several anecdotal cases of successful off-label use, so although there are physiological reasons for each contraindication, they should not prevent the use of this therapy in life-threating situations when there are few other options[3].