ABSTRACT
Bullous pemphigoid is a chronic autoimmune blistering disease. Recently, several reports suggested dipeptidyl peptidase 4 (DPP-4) inhibitors, also known as gliptins, were a potential cause of drug-induced bullous pemphigoid but not of both bullous pemphigoid and alopecia areata together. Here we describe the case of a 68-year-old man with type 2 diabetes mellitus who developed new onset diffuse alopecia on the scalp with diffuse tense bullae over his body a few months after linagliptin was introduced for better control of his diabetes.
DPP-4 inhibitors are not known to increase the risk of alopecia. To the best of our knowledge, this is the first report of linagliptin-associated alopecia areata and bullous pemphigoid, which may help demonstrate if there are any links between DPP-4 inhibitors and alopecia.
LEARNING POINTS
- This is the first report of linagliptin-associated alopecia areata and bullous pemphigoid (BP), which may help demonstrate a link between DPP-4 inhibitors and alopecia.
- ISince the time of onset of BP after initiation of a DPP-4 inhibitor varies, a high index of suspicion is needed for diagnosis.
- Early diagnosis is essential as DPP-4 inhibitor withdrawal has a significant effect on disease remission.
KEYWORDS
Gliptin, drug, bullous pemphigoid
INTRODUCTION
Bullous pemphigoid (BP) is an autoimmune disease where autoantibodies target structural proteins at the dermal–epidermal junction. Two hemidesmosomal proteins, 230 kDa protein and 180 kDa antigen, have been identified as the major targets of BP autoantibodies. BP manifests with tense blisters on the skin[1]. It is poorly understood although many trigger factors have been identified, such as contrast material injection, surgical procedures, mechanical trauma, insect bites, thermal burns, radiotherapy and ultraviolet exposure associated with pre-existing psoriasis[2]. Linagliptin is one of the new dipeptidyl peptidase-4 (DPP-4) inhibitors used in the treatment of type 2 diabetes mellitus (DM). DPP-4 inhibitors have been recently implicated in inducing BP, but the mechanism is not entirely clear. DPP-4 inhibitors may induce anti-basement membrane zone antibodies or other structurally similar antibodies, leading to sub-epidermal bullae and BP[3]. Many recent case reports show that use of DPP-4 inhibitors is a risk factor for BP onset, but there is no evidence of an association with alopecia.
CASE DESCRIPTION
A 68-year-old Caucasian man with a complex medical history including type 2 DM presented to the emergency department with a 3–4-week history of generalized pruritus, new onset diffuse alopecia and diffuse bullae over his trunk, arms and legs. The patient initially had developed bullae and blisters over his legs. Simultaneously, he noticed a significant loss of his scalp and beard hair as well as his eyebrows. This was accompanied by intense pruritus over the abdomen and back for approximately 2 weeks prior to the development of the bullae. The intense itching, development of further bullae, and almost complete alopecia prompted the patient to present to the emergency department.
A review of his history did not reveal any drug allergies and he denied a family history of autoimmune conditions. He had not travelled anywhere recently and did not present with any constitutional symptoms or myalgias. His home medications included linagliptin, allopurinol, amlodipine, atorvastatin, furosemide, hydralazine, levothyroxine, pantoprazole, rivaroxaban, terazosin and insulin.
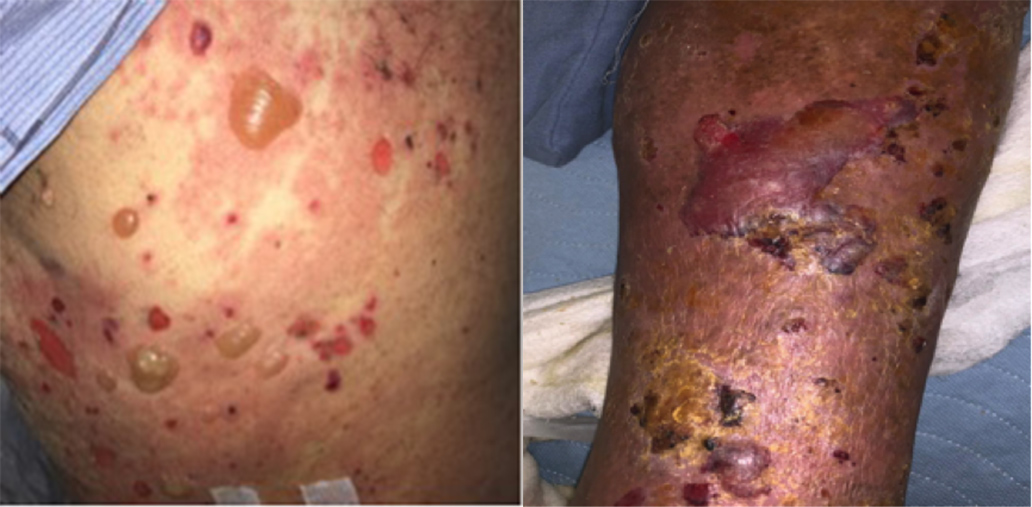
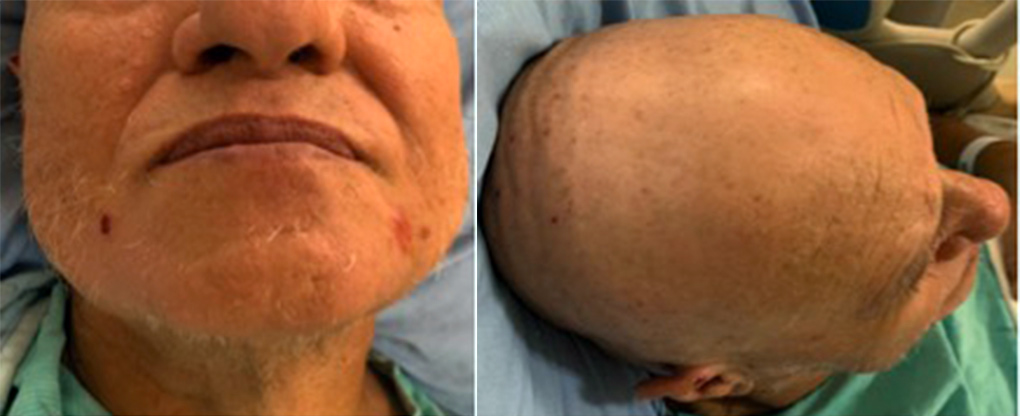
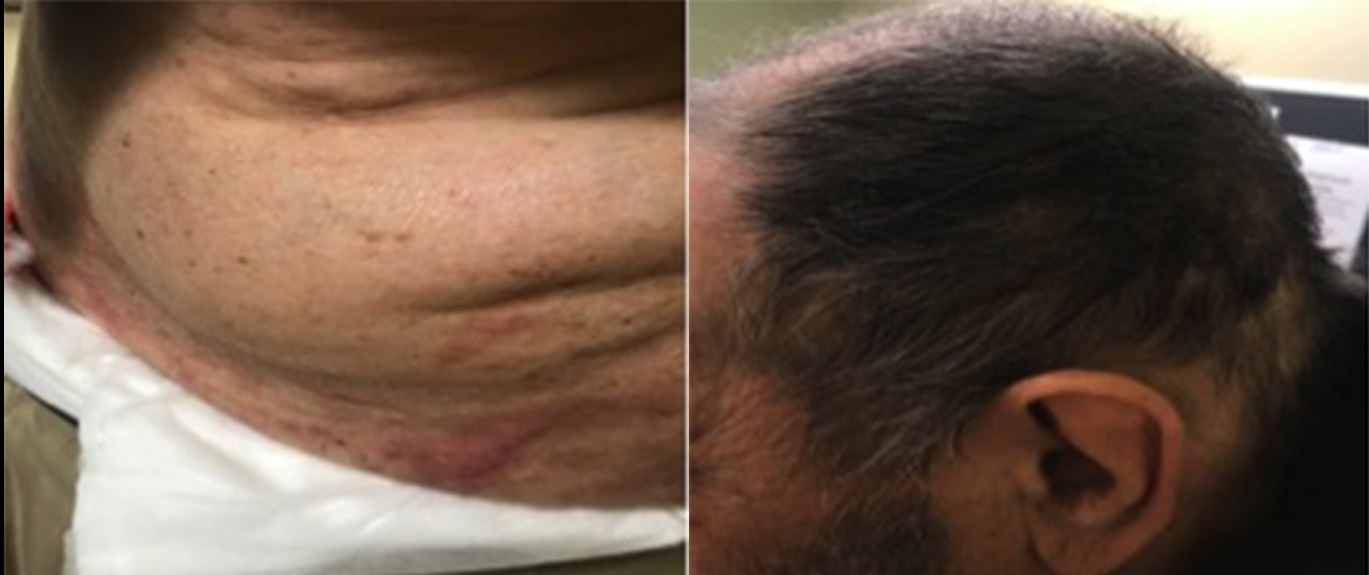
His vital signs were all within normal limits: he was afebrile at 36.8°C, his heart rate was 59 bpm, blood pressure was 118/73 mmHg, and oxygen saturation was 98% on room air. Physical examination revealed bullae over his back, abdomen and both lower legs and measuring approximately 1–3 cm in diameter (Fig. 1). He also had numerous smaller bullae over both flanks, upper arms and lower legs and measuring approximately 0.5–1 cm in diameter. There was no specific dermatomal distribution and no oral ulcerations or mucositis. The patient had diffuse alopecia on his scalp, eyebrows and beard. There was no erythema, scaling or scarring associated with the hair loss (Fig. 2).
Figure 1. Diffuse tense bullae and blisters on the patient’s initial presentation to hospital
Figure 2. Diffuse alopecia on the scalp and jaw with no erythema, scaling or scarring
Investigations
Initial laboratory investigations revealed an elevated creatinine level of 300 mmol/l (baseline in the mid-200s) with normal electrolytes. The patient also had an elevated CRP level of 12.6 mg/l, his white blood cell count was normal at 10.3 g/l, and haemoglobin concentration was 110 g/l.
Hospital course
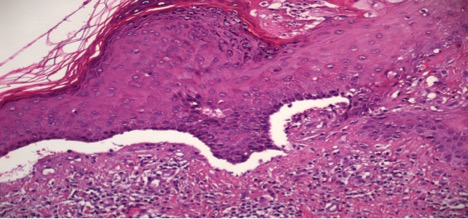
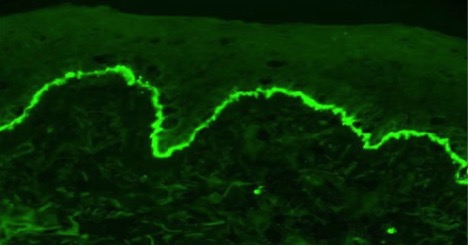
Skin biopsies performed on admission showed sub-epidermal blisters with multiple eosinophils highly suggestive of BP (Fig. 3). Eosinophils are commonly seen in BP, but the diffuse eosinophilia seen in the biopsy specimen raised the possibility of drug-induced BP. Direct immunofluorescence showed deposition of IgG and C3 along the basement membrane confirming BP (Fig. 4). Interestingly, linagliptin had been introduced a few months before the onset of cutaneous eruptions. At that point, linagliptin was highly suspected as the cause of BP and alopecia and therefore was discontinued. The patient was started on prednisone 40 mg with significant improvement of his skin lesions. He was then followed by the dermatology department and was started on mycophenolic acid (720 mg by mouth twice a day as a steroid-sparing agent) and topical clobetasol cream. At his 6-month follow-up, 80–90% of his skin lesions had resolved and his hair had grown back (Fig. 5A,B).
Figure 3. Sub-epidermal blister with multiple eosinophils
Figure 4. Direct immunofluorescence revealed deposition of IgG and C3 along the basement membrane
Figure 5. Almost complete remission of skin lesions and alopecia 6 weeks after treatment
DISCUSSION
BP is not uncommon and has an incidence of 0.2–3/100,000 person-years with a higher incidence in older age groups. A UK study estimated the incidence there was 1.4/100,000 person-years[4]. There is a wide variation in mortality rate, with a 1-year mortality rate of 13–41% in Europe and 11–23% in the USA[5]. The classic manifestation of BP is diffuse tense blisters, but clinical presentation can also include diffuse urticarial or dermatitis plaques[6].
DPP-4 inhibitors are a class of oral hypoglycaemic agents which can be used to treat type 2 DM. DPP-4 inhibitors work by increasing glucagon-like peptide-1 and glucose-dependent insulin-trophic polypeptide which leads to increased insulin and inhibits glucagon.
Sitagliptin was the first agent of this class to be approved by the FDA in 2006[7]. The DPP-4 enzyme has many functions including biological roles in pro-inflammatory pathways[8]. DPP-4 is also known as CD26 and is widely expressed in various cell types throughout the body including the skin, but the role of the DPP-4 enzyme in autoimmune pathogenesis has not yet been clearly elucidated[9,10]. A large observational study has indicated that the use of DPP-4 inhibitors is associated with an overall 75% increase in the risk of inflammatory bowel disease in patients with type 2 DM[9]. However, another large observational study indicated that DPP-4 inhibitor combination therapy appears to be associated with a decreased risk of autoimmune diseases, including rheumatoid arthritis, compared with non-DPP-4 inhibitor combination therapy[11].
A few months after starting linagliptin, our patient developed alopecia areata totalis and a skin eruption shown to be BP by histological examination and immunofluorescence. Several cases of linagliptin-induced BP have been reported (Table 1. However, none of those cases described both alopecia areata and BP simultaneously due to linagliptin.