ABSTRACT
Iatrogenic antineutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) is not exceptional. Many cases of small vessel vasculitis induced by anti-thyroid drugs (ATD), mainly propylthiouracil (PTU), have been reported. We present a case of AAV related to another ATD: benzylthiouracil (BTU) and review the literature. An 84-year-old man with a 4-year history of multinodular goitre with hyperthyroidism was treated with BTU. He presented an acute syndrome with weakness, fever, epigastric pain and abdominal distension. Lactate and lipase tests were normal. An abdominal scan showed a thrombosis of the splenic artery with splenic infarction. We excluded a hypothesis of associated embolic aetiology: atrial fibrillation, atrial myxoma, intraventricular thrombus or artery aneurysm. Exploration of a possible prothrombotic state (complete blood count, haemostasis tests, activated protein C resistance, factor V Leiden, protein C, S, antithrombin III) gave normal results. Tests for antinuclear antibodies (ANA) and antiphospholipid antibodies (APL) were negative. However, testing for p-ANCA, with antimyeloperoxidase (MPO) specificity, was positive: 120.6 CU (N<20.0). We did not find other systemic manifestations, except a non-specific kidney failure. BTU was discontinued without steroids or immune-modulating drugs. Subsequently, symptoms disappeared progressively and titres of ANCA fell until normalization, 4 months later. Many patients treated with BTU present a high prevalence of ANCA, mainly, but not exclusively, directed against MPO. Vasculitis, however, remains an uncommon complication. The mechanism of this anomaly remains to be elucidated. Some studies suggest the possibility of an autoimmune reaction initiated by drug bioactivation mediated by neutrophil-derived MPO. The present observation is particular because the involved drug was BTU and clinical expression was unusual.
LEARNING POINTS
- ANCA-associated vasculitis related to anti-thyroid drugs is not exceptional, particularly in patients receiving long-term therapy with thioamides.
- Clinical manifestations are highly variable.
- Treatment consists firstly of stopping the anti-thyroid drug. Introduction of steroids and/or immunosuppressive therapy depends on the severity of organic impairments. Prognosis is less severe than primary ANCA vasculitis.
KEYWORDS
Anti-thyroid drugs, benzylthiouracil, ANCA vasculitis, hyperthyroidism
INTRODUCTION
Antineutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) is a group of small vessel systemic vasculitides. ANCAs were first discovered in 1982 by Davies et al. in patients’ sera with pauci-immune segmental necrotizing glomerulonephritis[1]. ANCAs are autoantibodies targeted against antigens present in the cytoplasm of neutrophils and monocytes. The most common target antigens are proteinase 3 (PR3) and myeloperoxidase (MPO)[2]. Anti-thyroid drugs (ATD) are widely used in the treatment of thyroid disorders. Adverse effects related to the use of this medication include agranulocytosis, cutaneous rash, toxic hepatitis and induced lupus-like syndrome. AAV is not an exceptional complication. It has mainly been reported with propylthiouracil (PTU)[3]. We present here a case of ANCA-anti-MPO-associated vasculitis related to another thioamide: benzylthiouracil (BTU).
CASE DESCRIPTION
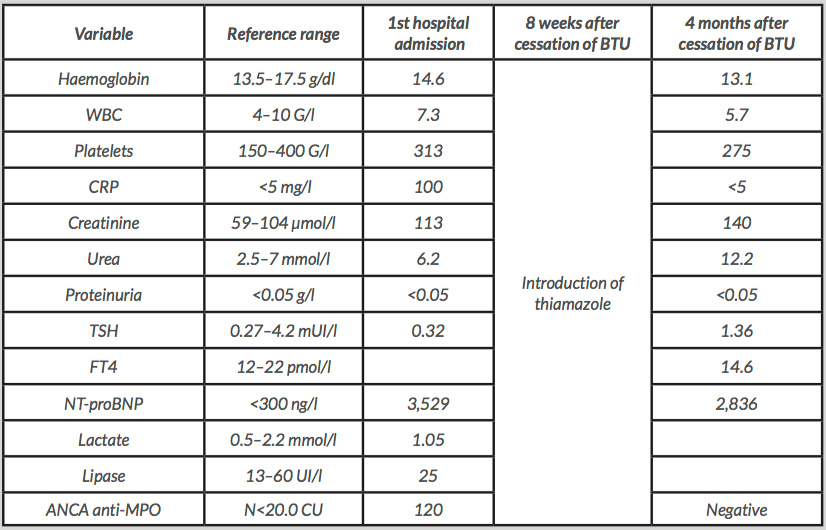
An 84-year-old male with a clinical history of multinodular goitre and hyperthyroidism presented an acute syndrome with fever and epigastric pain. Past history included hypertension, chronic obstructive airways disease and benign prostatic hyperplasia. Thyrotoxicosis was diagnosed 4 years previously. FT4 was 23.2 pmol/l (12–22 pmol/l), TSH was <0.005 mUI/l (0.27–4.2 mUI/l) and TSH receptor antibodies were negative. The patient had been treated and well controlled on BTU 100 mg/day. On admission, blood pressure was 160/82 and his pulse was regular (88/min). The patient was complaining of an epigastric pain with important abdominal distension and a rebound tenderness at the left upper quadrant. A small multinodular goitre was palpable. The obtained laboratory data are shown in Table 1. An abdominal scan showed a distal thrombosis of the splenic artery with splenic infarction. There was no artery aneurysm. An ECG showed a left bundle branch block with lateral repolarization abnormalities. There were no occasional cardiac arrhythmias or atrial fibrillation on Holter monitoring. Echocardiography did not find a thrombus in the left ventricular cavity, no arguments for an endocarditis, but did show an anteroseptal akinesis. The laboratory exploration of a possible prothrombotic state: complete blood account, haemostasis tests, activated protein C resistance, factor V Leiden, protein C, S, antithrombin III, showed normal results. A HIV serology test was negative. We detected a moderate hyperhomocysteinaemia of 21.8 (N:5–15 μmol/l). Testing for antinuclear antibodies (ANA), antiphospholipid antibodies (APL) and ANCA anti-PR3 was negative. However, testing for p-ANCA, with anti-MPO specificity, was clearly positive: 120.6 CU (N<20.0). We did not find other systemic manifestations, except a kidney failure stage 3A, without haematuria or proteinuria. BTU was discontinued without steroids or immune-modulating drugs. Subsequently, clinical symptoms progressively ceased. Two months later, thiamazole 10 mg was introduced because of a relapse of the thyroid disorder.
Four months after the cessation of BTU, titres of ANCA-MPO fell until normalized. Serum concentrations of TSH and FT4 were appropriate, and creatinine was stabilized at 140 μmol/l.
SUMMARY OF CASES
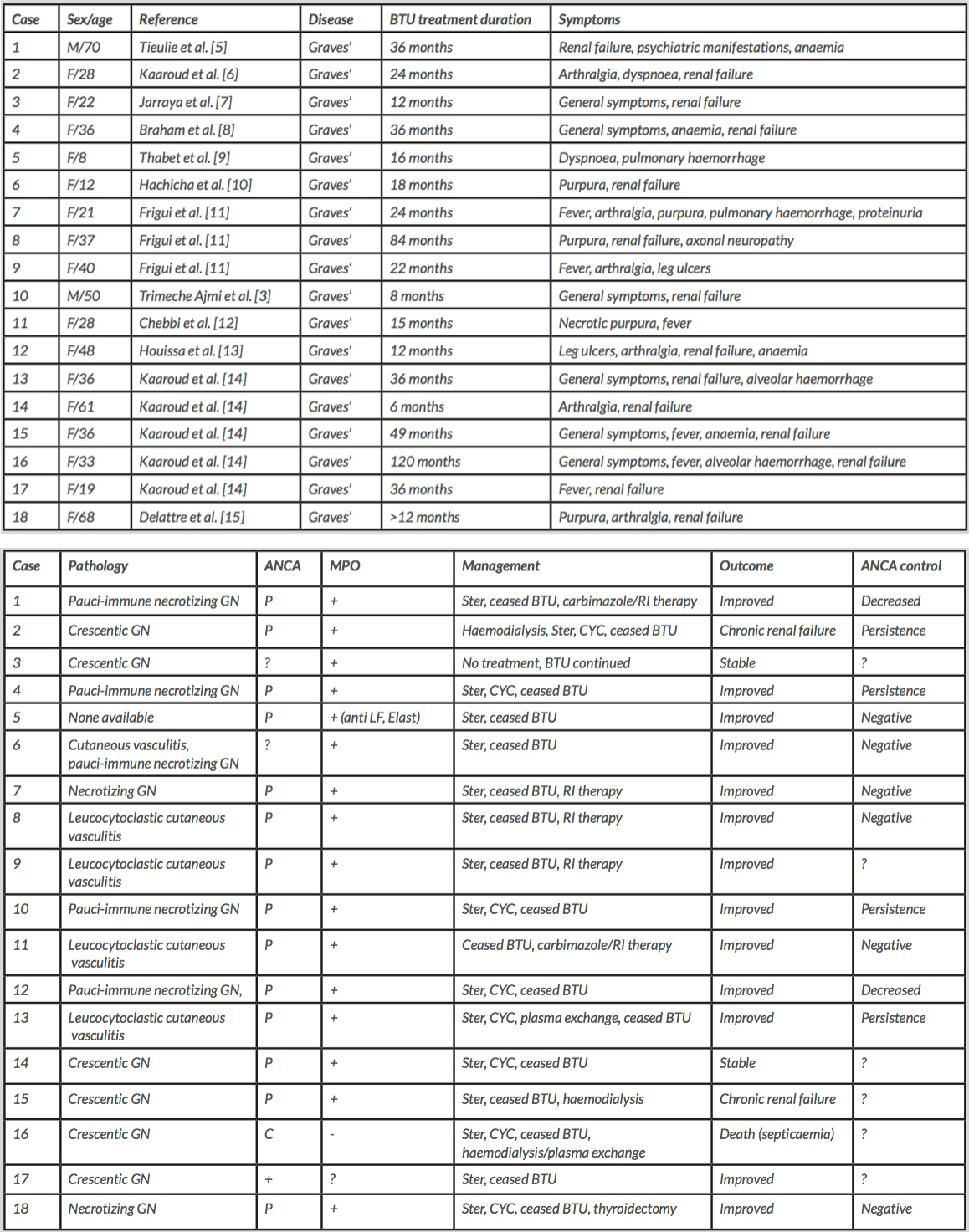
The first induced AAV case was reported in 1992 by Stankus and Johnson, and was related to PTU[4]. In 2002, Tieulie et al. described the first case caused by BTU[5]. Since then, 17 other observations of induced AAV related to BTU have been reported (see Table 2).
89% of cases were female,probably reflecting the female preponderance of thyrotoxicosis. The average age of affected patients was 38.8 years (range 8 to 84 years). All of patients presented Graves’ disease,while the average duration of exposure to BTU was 32 months (range 6 to 120 months).
Renal involvement was the most common manifestation (83%), followed by general symptoms (fever, weight loss, anorexia, asthenia) in 56% of patients, skin manifestations in 39%, joint pain in 33%, pulmonary vasculitis with alveolar haemorrhage in 22%, with neurological/neuropsychiatric manifestations having been reported in two cases (11%). Renal biopsy showed necrotizing glomerulonephritis in 8 cases (44%) or crescentic glomerulonephritis in 6 (33%). In cases of skin involvement, biopsy revealed a non-specific leucocytoclastic cutaneous vasculitis. Immunofluorescence was always pauci-immune.
A p-ANCA pattern was present in 78 %, whilec-ANCA was seen in only one case (#16). Undifferentiated positive ANCAs were reported in 3 observations (17%). In 89%, ANCAs were directed against MPO. PR3-ANCAs were negative in all sera, when tests were performed.Therapy consisted ofstopping BTU. Additional treatment with steroids and/or cyclophosphamide was initiated in patients presenting severe organic impairment (89%). In 72%, therapy resulted in improvement. Concurrently,titresof ANCAs decreased or disappeared progressively but persisted in some cases (#4, #10, #13). In 11%, renal function declined (#2, #15). Death occurred in one case related to Klebsiella pneumoniae septicaemia (#16).
DISCUSSION
Our 84-year-old patient had been treated with BTU for 4 years. He did not present severe organic impairment but had sudden pain and fever, with detection of splenic artery thrombosis within a positive ANCA-MPO context. Exclusion of other aetiologies and resolution of symptoms after discontinuing BTU suggested a close relationship between the ATD and clinical manifestations. Exposure duration to BTU in our case was long. According to Gunton et al., there is a significant association between duration of therapy, mainly with PTU, and ANCA positivity (p<0.0001). Testing patients receiving long-term anti-thyroid medication seems to be interesting[16]. A p-ANCA pattern with anti-MPO specificity seems to be the most common in BTU-induced AAV. To our knowledge, a single abdominal vascular involvement has never been observed previously. Manifestations of AAV related to ATD are variable; they may consist of non-specific constitutional symptoms[17] or may involve vessels in the skin, kidneys, respiratory tract or peripheral nerves[18]. The prevalence of ANCA in patients treated with ATD varies from 4 to 46% while the prevalence of AAV is lower: 0–1.4%. Slot et al. demonstrated that ANCA positivity was significantly related to the use of ATD[19]. The presence of ANCAs without vasculitis manifestations suggests that ANCAs alone are not enough to induce vasculitis. Furthermore, high ANCA titres may persist without activation of vasculitis. The pathogenic role of ANCA-MPO in vasculitis seems to be related to sub-classes of anti-MPO antibodies[20].The mechanism by which the ATD, and particularly, thiouracils, may induce AAV remains to be elucidated[8]. Jiang et al. showed that PTU, among other medications, was highly cytotoxic in the presence of activated neutrophils[21]. Treatment depends on vasculitis localization and clinical severity. Minor symptoms respond well to cessation of the ATD. In cases of seriousrenal damage, treatment with steroids with or without cyclophosphamide should be considered. In cases of life-threatening pulmonary haemorrhage, in addition to steroids and immunosuppressive drugs, plasmapheresis is warranted[18]. Derivatives of imidazole are preferred, in cases of relapse, before considering a radical treatment involving surgery or radioactive iodine therapy. Prognosis is less severe than primary ANCA vasculitis, and death due to anti-thyroid therapy-induced AAV is exceptional, related generally to severe alveolar haemorrhage[22].