ABSTRACT
Introduction: Haemoglobin A1C (A1C), as a parameter of long-term glycaemic control, has been adopted to guide diabetic therapy all over the world. However, falsely high or low A1C could be troublesome in daily practice.
Case description: A 75-year-old male diabetic patient affected by a reasonably inferred life-long history of microcytic anaemia was found to have abnormally low A1C values in the previous 5 months. Subsequent laboratory assessment with brilliant cresyl blue staining and haemoglobin electrophoresis detected haemoglobin H disease as the underlying cause of both the microcytic anaemia and the disturbed A1C measurement.
Discussion: Enhanced erythrocyte destruction such as in haemoglobin H disease could explain a falsely decreased A1C level very well. Upon facing a questionable A1C value, physicians dealing with diabetes should consider the possibility of undiscovered underlying causes rather than too tightly glycaemic control.
LEARNING POINTS
- Haemoglobin A1C values lower than the normal range most likely do not mean too good a control of blood sugar in diabetic patients.
- Careful investigation to find the underlying causes is mandatory to provide well-qualified medical care.
- Various haemoglobinopathies with chronic haemolysis should be considered as the background reason, especially in an endemic area for thalassaemia.
KEYWORDS
Haemoglobin A1C, haemoglobin H disease, microcytic anaemia, thalassaemia
INTRODUCTION
Haemoglobin HbA1C (A1C) levels have been widely recognized as being a reliable estimate of long-term blood sugar levels, particularly in evaluating the efficacy of glycaemic control in diabetic patients. Nevertheless, abnormally increased or decreased A1C levels may be detected due to various underlying pathologic causes, chiefly including altered erythropoiesis rates, erythrocyte destruction, haemoglobinopathy, alcoholism, chronic renal failure, splenomegaly, hyperbilirubinaemia, hypertriglyceridaemia and certain drugs[1]. Herein, we would like to present a case involving an elderly diabetic patient with moderately severe microcytic anaemia and persistently low A1C levels which had been initially misunderstood as representing over-strict glycaemic control. Disclosing the underlying cause of abnormally decreased A1C levels may act as a reminder to the physician in charge of the necessity of using alternative tests other than A1C measurement in guiding diabetic management.
CASE DESCRIPTION
A 75-year-old man was brought to our outpatient clinic from a nearby nursing home with the chief complaint of progressive dizziness and weakness for several weeks. Fever, nausea, vomiting, abdominal pain, dark urine, haematemesis, melaena and haematochezia were all denied.
The patient had been on regular medication for medically controlled hypertension and gouty arthritis in the past 2 years. Type 2 diabetes was diagnosed based on elevated fasting and postprandial plasma sugar levels 1 year previously. Dapagliflozin and repaglinide had been prescribed ever since. His surgical history included appendectomy and fixation of a right femoral intertrochanteric fracture more than 8 years previously.
On physical examination, a pale conjunctiva and tachycardia, 111 regular beats per minute, with mild systolic murmur, were the most notable findings. The blood pressure was 116/55 mmHg and the respiratory rate was 20 per minute with clear breathing sounds. The sclera was not icteric.
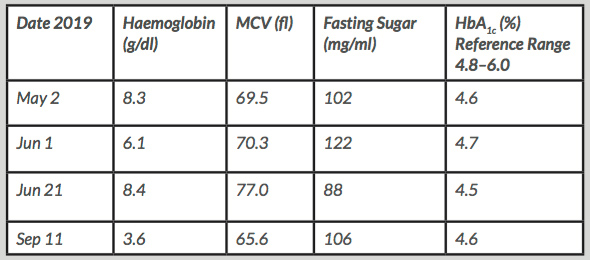
A routine blood test showed white blood cells at 3,000/µl, thrombocytes at 187,000/µl, haemoglobin of 3.6 g/dl, mean corpuscular volume (MCV) of 65.6 fl and red cell distribution width of 38.5%. Biochemical evaluation revealed uric acid at 7.8 mg/ml, fasting plasma sugar at 106 mg/ml and normal liver and renal function. Interestingly, A1C was only 4.6% (reference range 4.8%–6.0%). The serum iron concentration was 157 µg/dl (reference 33–193), total iron binding capacity was 183 µg/dl (reference 245–419) and ferritin levels were 1,423.06 ng/ml (reference 21.81–274.66). Antibodies against hepatitis B virus surface antigen were positive and antibodies against hepatitis C virus were negative. Alpha-fetoprotein, carcinoembryonic antigen, cancer antigen 19-9 and prostate specific antigen were all within normal limits. Occult blood in stool and urine samples was negative. There was also no microhaematuria.
A sonogram of the abdomen found essentially normal biliary trees, mild coarsening of liver parenchyma, compatible with chronic parenchymal liver disease, and splenomegaly. Upper gastrointestinal tract endoscopy disclosed mild mucosal hyperaemia and some erosion over the antrum without active bleeding foci.
The patient’s general condition improved to a great extent after red blood cell transfusion therapy. Nonetheless, tracing his medical record led to the finding of an at least 10-year-long history of persistent microcytic anaemia frequently rescued with red blood cell transfusions. Furthermore, A1C values constantly below the lower reference limit accompanying sometimes slightly elevated fasting plasma sugar levels in the past 5 months were noted (Table 1). Importantly, the patient had never had symptoms related to hypoglycaemic attacks.
Table 1. Fasting plasma sugar levels and corresponding haemoglobin A1C values
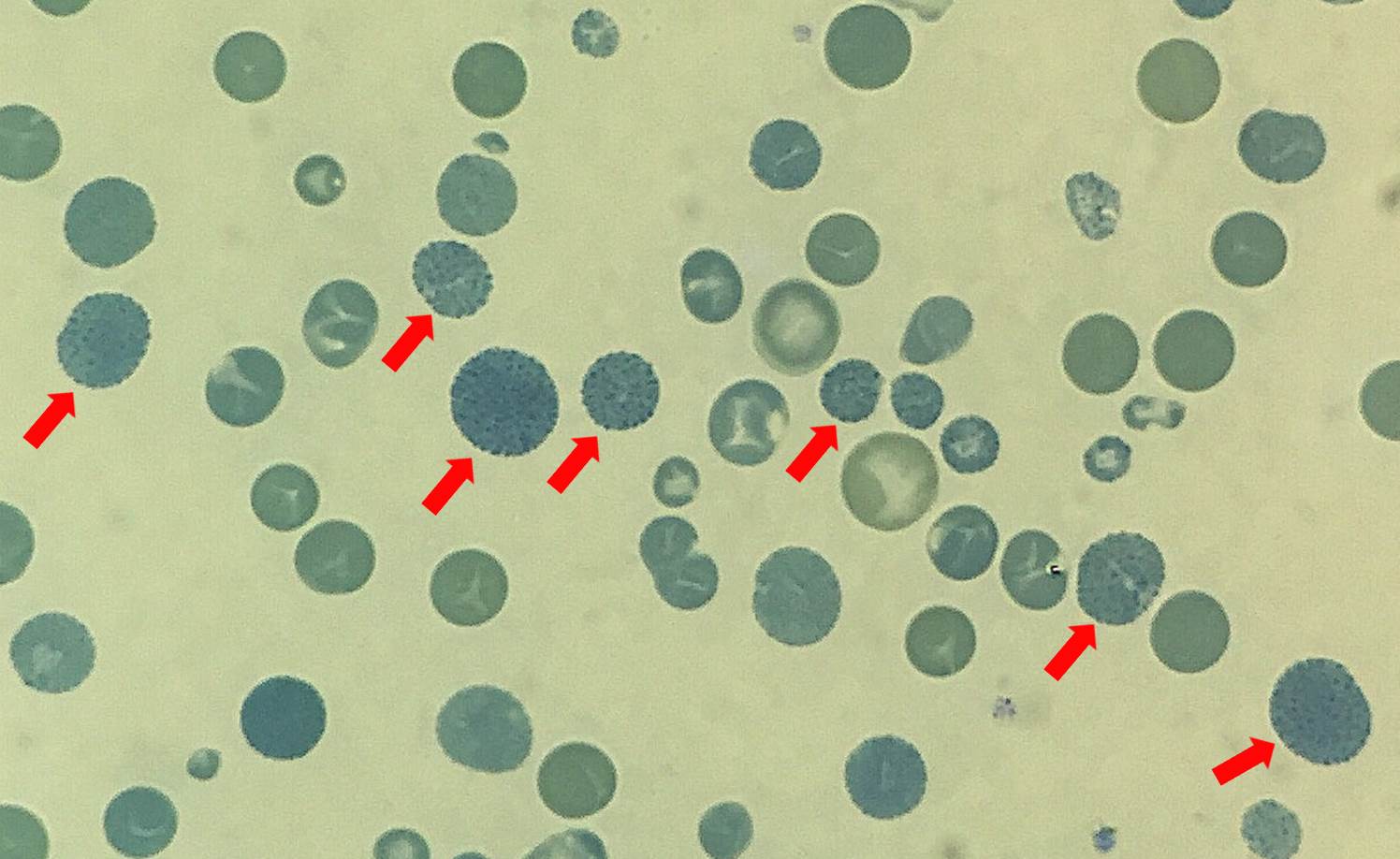
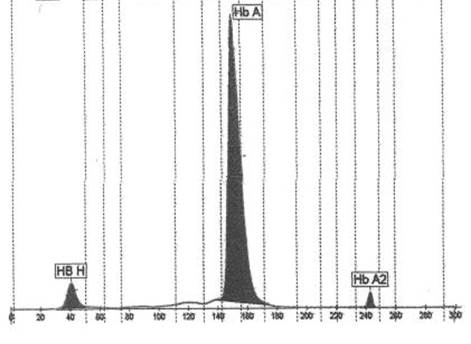
To investigate the cause of the incompatibility between the A1C and blood sugar levels, haemoglobin H (HbH) staining using brilliant cresyl blue and haemoglobin electrophoresis were carried out. Unsurprisingly, many erythrocytes containing golf ball inclusions appeared in the HbH staining smear (Fig. 1). Furthermore, a small HbH peak was detected following haemoglobin electrophoresis, estimated to occupy 6.2% of the total haemoglobin content (Fig. 2). Thus, HbH disease, a moderately severe form of alpha thalassaemia with 3 silent alpha globin genes, was diagnosed to be the underlying cause of the falsely low A1C.
Figure 1. Haemoglobin H staining with brilliant cresyl blue. Arrows: erythrocytes with golf ball inclusions
Figure 2. Haemoglobin electrophoresis chromatography. Haemoglobin A 92.1%, haemoglobin A2 1.7%, haemoglobin H 6.2%
DISCUSSION
Although the main differential diagnoses of microcytic anaemia include iron deficiency and thalassaemia, iron deficiency can definitely be ruled out based on a high serum ferritin level, low total iron binding capacity and no evidence of blood loss in this patient. In addition, many previous studies have found that the level of A1C is usually falsely increased rather than falsely decreased in iron deficiency[2], further supporting thalassaemia as a more likely diagnosis.
Clinically, a rapid and confident diagnosis of alpha thalassaemia often relies on brilliant cresyl blue staining because HbH is unstable and tends to decay during haemoglobin electrophoresis[3]. Hence, the patient’s measurement of 6.2% HbH may also be underestimated. However, we are glad to uncover an obvious mystery hiding behind a paradoxical phenomenon.
Despite a former case report providing evidence that falsely high A1C could be observed when HbH was superimposed onto the A1C peak in ion exchange high pressure liquid chromatography analysis[4], a recent investigation concluded that A1C values were significantly lower in patients with HbH disease than in control individuals and patients with only 1 or 2 silent alpha globin genes[5]. The opposite results seem to stem from different analytic devices used.
In conclusion, physicians must be aware of the potential interference from haemoglobinopathies with enhanced erythrocyte destruction in A1C measurement among individuals from an endemic area for thalassaemia.