ABSTRACT
Chemotherapy usually causes complications affecting several tissues such as oral mucosa. In this case report, a soft palate oral ulcer caused by chemotherapy was treated by ozone gas. This kind of treatment is known for its antimicrobial, regenerative and analgesic proprieties. The results show a complete resolution of the lesion within 2 weeks of treatment. Ozone therapy demonstrates greater effectiveness with respect to this kind of oral lesion compared to traditional therapy. Considering this evidence, ozone therapy should be considered as a useful tool for the adjuvant therapy of oral complications in oncologic patients.
LEARNING POINTS
- Intensive chemotherapy can have side effects, particularly affecting tissue with higher turnover. Therefore, there is a clinical need to prevent or to treat such complications.
- Ozone therapy could improve oral mucosa healing and have anti-inflammatory, antioxidant and antibacterial effects to prevent suprainfections. To date, there are no reported cases of oral ulcers in oncologic patients being completely resolved using ozone in the literature.
- Medical and dental doctors should collaborate with regards to complex patients to prevent such types of complications, discovering these clinical cases that are unknown in the literature and treating patients in a more comprehensive way.
KEYWORDS
Ozone therapy, leukaemia, adjuvant therapy, oral ulcer, oral fungal infections, ozone, dentistry
INTRODUCTION
The treatment for acute lymphocytic leukaemia (ALL) in adults is usually long-term chemotherapy. Intensive chemo regimens are undertaken to obtain better clinical responses, but may also lead to complications and side effects. In these patients, if the immune system is damaged, opportunistic infections can occur and, sometimes, complications can be serious enough to be life-threatening. Today, thanks to the multidisciplinary approach regarding supportive care (nursing care, nutrition, antibiotics, growth factors and so on) for oncologic patients, these life-threatening episodes are far less common than in the past[1].
Side effects of chemotherapy affect rapidly proliferating cells, such as in the bone marrow, intestinal mucosa, oral mucosa, hair follicles and gonads. In particular, in oral mucosa, basal cells are lysed, replacement and turnover slow down resulting in mucosal ulcerations, or other oral damage such as aphthous lesions, mucositis and oral candidiasis caused by immunodepression[2].
Ozone is a gas known for its antibacterial, antiviral and antifungal properties and it is widely used in medicine and dentistry[3]. Medical ozone can improve the microcirculation and it has anti-inflammatory, analgesic and immune-modulating properties. All of these characteristics qualify medical ozone as a valid candidate within clinical dentistry, especially for the treatment of soft tissues.
From a pharmacological point of view, ozone therapy follows the principle of hormesis: it has high efficacy with lower concentrations, but at a greater dosage, it can be ineffective, or even toxic. At low dosage, this powerful oxidizing agent stimulates endogenous antioxidant activity and the production of interleukins and leukotrienes, causing a reduction in inflammation and pain.
In this article, an ulcerative lesion located in the soft palate has been treated by ozone as an adjuvant therapy in a leukaemic patient undergoing an intensive chemo regimen[4].
CASE REPORT
The patient was a 69-year-old male who was sent to the Tuscan Stomatologic Institute because of an oral lesion diagnosed as major aphthous ulcers that had not healed over 25 days. Anamnestic data were collected. The patient suffered from lymphoblastic Ph+ leukaemia (ALL) and was on his second chemotherapy cycle with monoclonal antibodies, namely, blinatumomab. The oral lesion was classified as an ulcerative lesion of the oral palatal tissue, probably due to the side effects of these therapies on the oral cavity.
The ulcerative lesion had already appeared 7 days after chemotherapy started, causing, at the beginning, just a light sting and then, after 25 days, it had developed into an extended ulcer. During these 25 days, antibiotic, antimycotic, analgesic and opioid medications had been prescribed to treat the lesion by haematologists and otorhinolaryngologists (FANS, opioids, amoxicillin and clavulanic acid, morphine, oxycodone, econazole, vitamins and oral gel specific for aphthosis).
The lesion did not heal; instead, it became chronic so the haematologist of the patient decided to suspend chemotherapeutical treatment until the lesion disappeared completely. This meant the lesion needed to be resolved as soon as possible.
The patient reported a constant and strong pain, similar to a jellyfish burn, which made it impossible for him to eat, drink and swallow, risking other systemic problems due to malnutrition. Consequently, the patient suffered from xerostomia caused by dehydration and drugs.
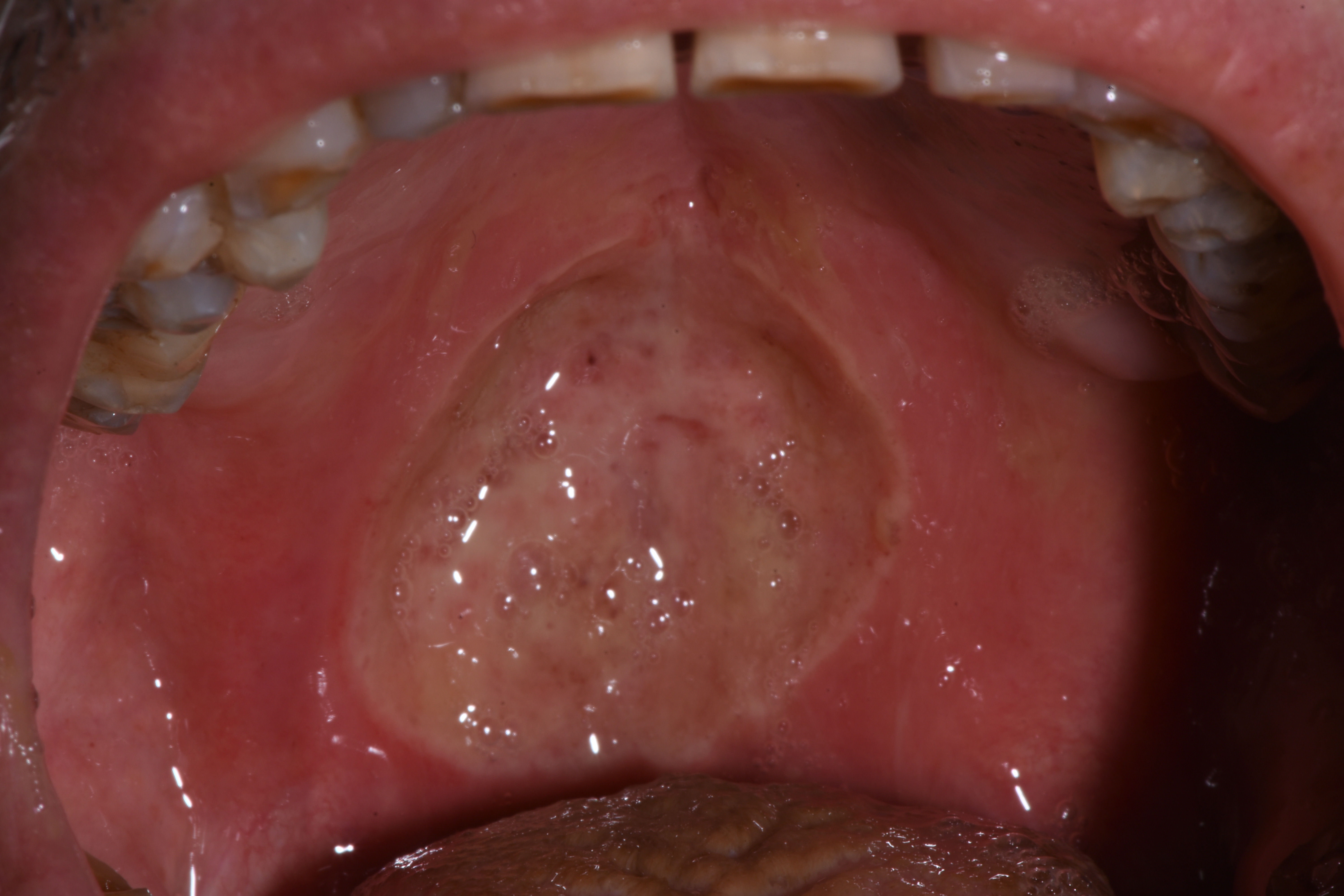
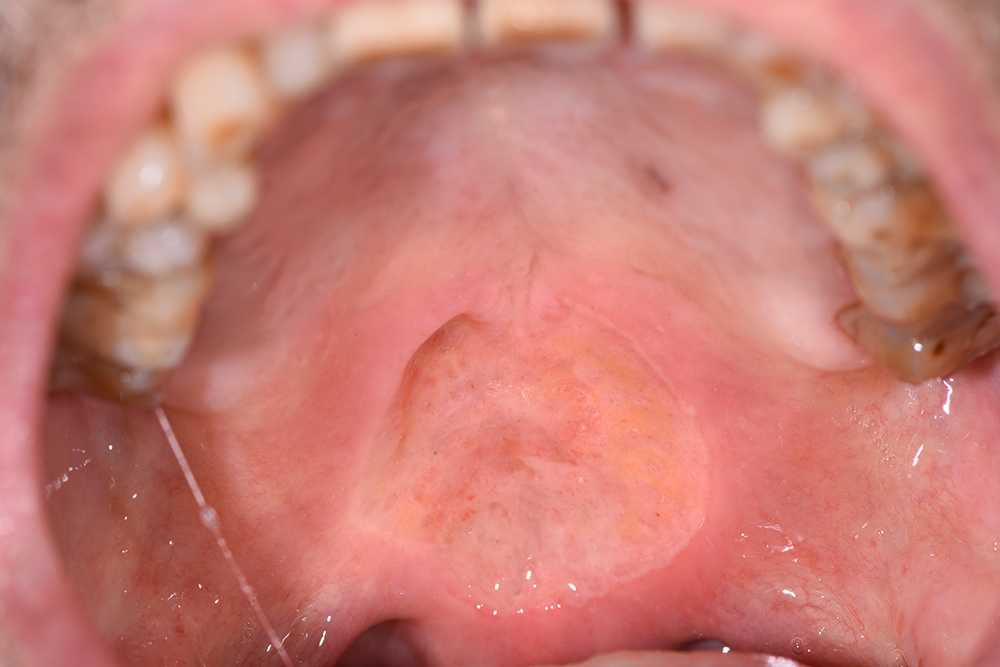
On first inspection at the Tuscan Stomatologic Institute (T0), that is, 25 days after the oral lesion had started, the ulcer (Fig. 1) located in the soft palate was more than 2 cm in diameter and several millimeters in depth. Above the lesion and on the back of the tongue there was a Candida suprainfection, which did not respond to antimycotic medication. The ulcer was treated using an ozone generator device (OZONE DTA, Sweden & Martina, Padua, Italy), which develops ozone from environmental oxygen. When ozone therapy started, all other treatments were interrupted except for antimycotic treatment of opportunistic infections. The patient could also still take analgesics (opioids or ketoprofen) when needed, and a sodium hyaluronic spray with amino acids (Aminogam, Polifarma Benessere, Italy) several times during the day.
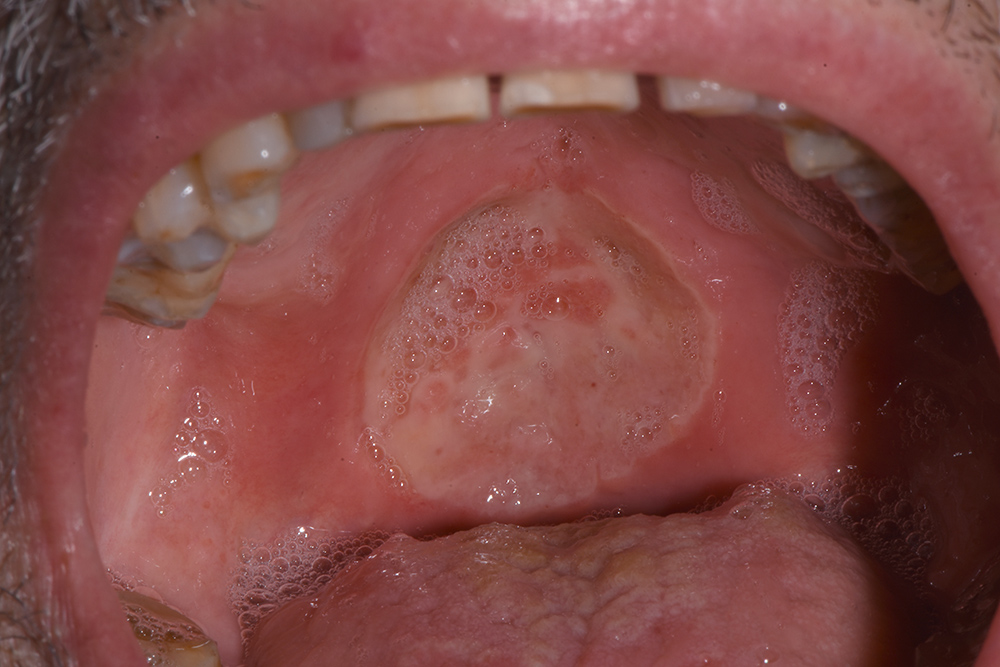
The first ozone session consisted of 5 applications of 2 minutes each, gradually increasing the power from 5 to 9. The insert utilized was the n #5 for mucosal use. The top of the probe in contact with the tissue released energy along the treated area. The ozone concentration was between 10 and 100 µg/ml. The patient felt pain during the first applications and the lesion was wetted with saline solution to reduce discomfort. At the end of the first session, the patient had already reported a reduction in pain, and he was able again to swallow and drink water. The patient returned after 2 days (T1). The diameter and the depth of the lesion had decreased, the colour was more natural (a pinkish colour) and the oedema was reduced (Fig. 2). After this second session, the production of saliva increased, the Candida infection was reduced and the patient stopped opioid intake, continuing with just ketoprofen once a day.
Figure 1. Initial photo, T0
Figure 2. T1, 2 days after the first applications
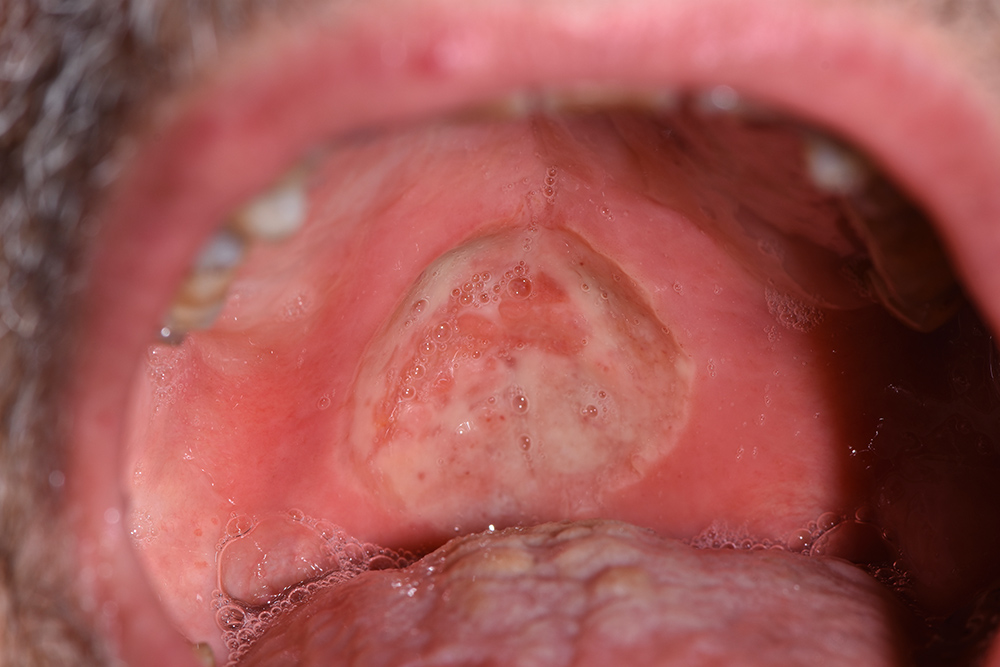
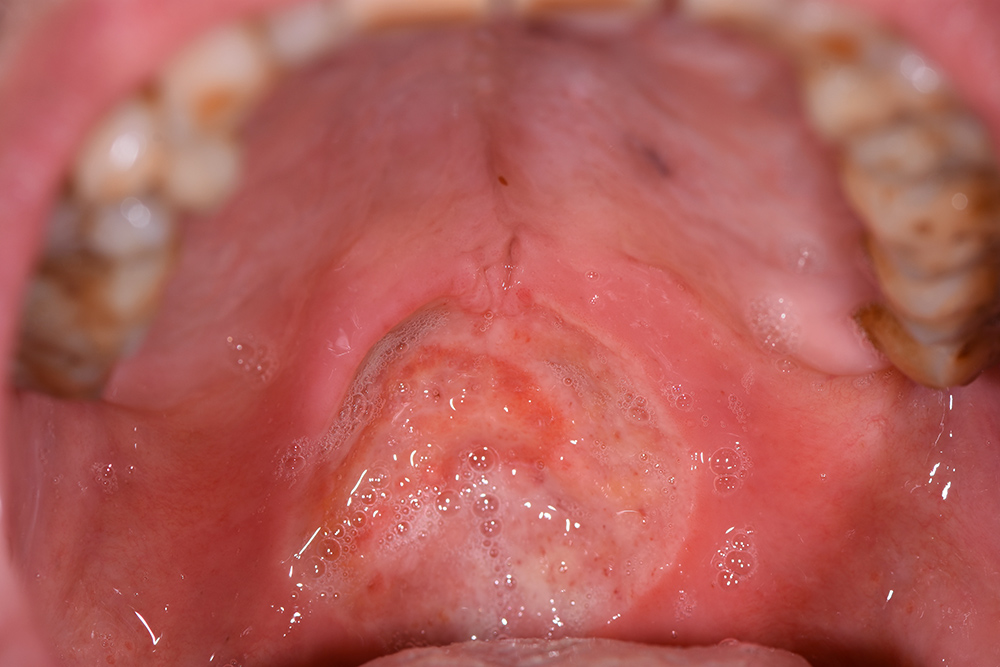
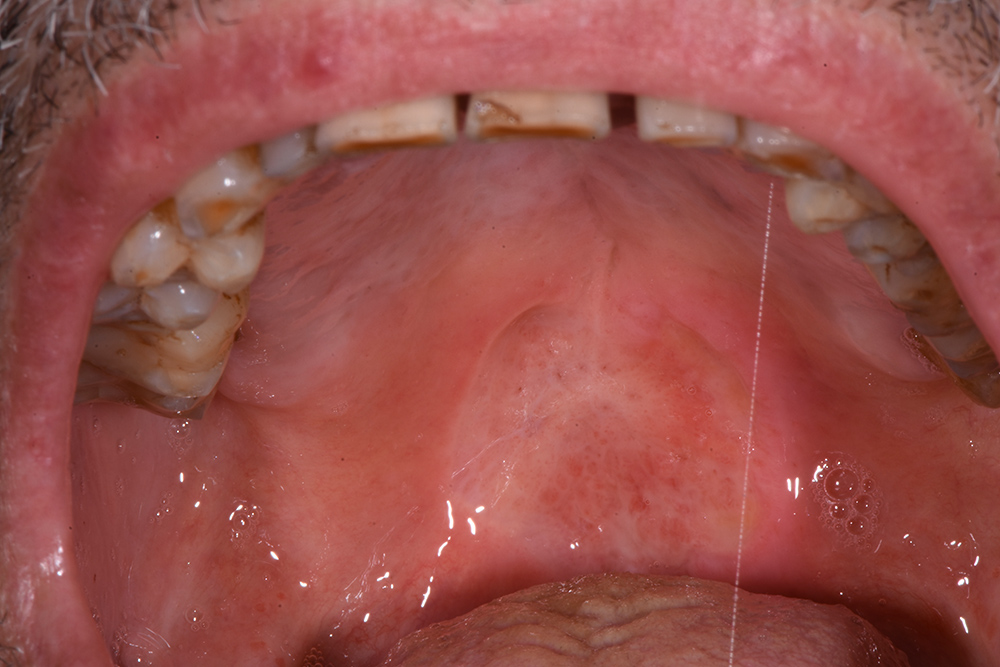
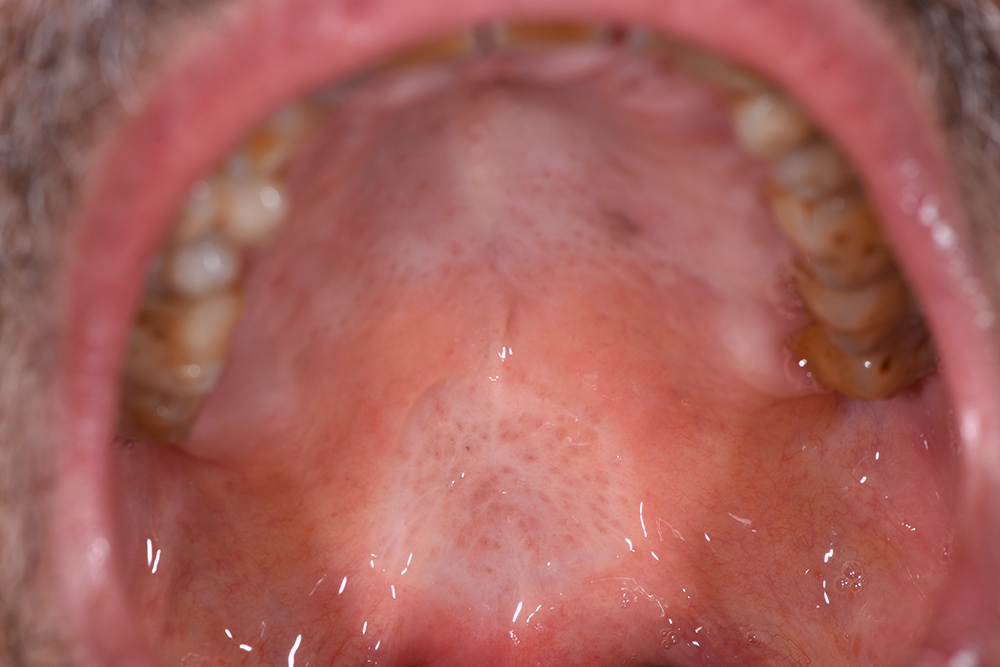
The treatment continued with ozone applications (10 minutes each) every 2 days, using the mucosal and angled insert, alternatively, in order to also reach the deepest zone of the soft palate (Fig. 3 and Fig. 4). Constant improvements were clinically appreciable and were noticed also by the patient. After 15 days, the lesion was remarkably reduced so doctors decided to restart the antitumoural therapy (Fig. 5). After 22 days (Fig. 6), the ozone treatment was ended after a last application of ozone gas with the same machine and ozonated water (2 cycles of 1.5 minutes using the Aquolab® Professional). After 1 month, the patient returned for monitoring of lesion healing (Fig. 7).
Figure 3. Four days after the first applications
Figure 4. Eight days after the first applications
Figure 5. Fifteen days after the first applications
Figure 6. Last applications of ozone, 22 days after the first applications
Figure 7. No treatment, follow-up 31 days after the first ozone applications
DISCUSSION
The most frequent oral complications of antitumoural therapy are mucositis, ulcerative lesions, oral infections, pain and salivary gland dysfunction[5]. In order to treat these kinds of lesions, oncologic patients are often monitored by other specialists to control complications before they become chronic. In some cases, the pharmacologic therapy may not be enough, and the patient may also need adjuvant therapy to facilitate the healing process, preventing correlated complications such as dehydration and malnutrition[6]. Ozone stimulates the release of interleukins and leukotrienes, reducing inflammation and promoting healing of the damaged tissues[7]. Ozone treatment causes nitric oxide release, which is a vasodilator. Nitric oxide improves the red cells tissue blood perfusion and stimulates aerobic processes such as glycolysis and the Krebs cycle[8,9]. Based on this evidence, ozone therapy was also shown to play an important role in reducing oral lichen planus symptoms[10].
Chlorhexidine was considered the gold standard for treating several infections and inflammatory diseases in the oral cavity but it has certain side effects, especially with prolonged treatment. Kaur and co-workers (2019) reported better results, even if not statistically significant, with ozonated water irrigation than with 0.2% chlorhexidine gluconate irrigation in the management of periodontal disease in 20 patients[11]. Other studies have reported encouraging effects of ozone irrigation combined with or as an alternative to chlorhexidine, because it may shift the oral microbiota to health[12,13].
According to a review by Kaimala et al. (2018), the oral microbiota could also help patients by acting as an immunotherapeutic agent modulating the immune system[14]. Furthermore, ozone has a high level of biocompatibility with epithelial cells, mucosal cells and fibroblast cells, along with its effects on other biological actions such as biosynthetic, haemostatic, bioenergetic, analgesic and antihypoxic processes, and these properties of ozone are refined upon contact with water (as in the oral cavity)[15,16].
This biological rationale and these clinical results encourage clinicians to collaborate for a multidisciplinary medicine where oncologic patients are also supervised by a dentist to monitor or prevent the complications of chemotherapy.
CONCLUSION
In this clinical case, an ulcerative lesion located in the soft palate of a patient undergoing chemotherapy was treated by ozone therapy. The initial pain and extensions of the lesion forced clinicians to interrupt the chemotherapy and start treating the lesions with antibiotic, antimycotic, analgesic and opioid medications, without evident improvements. Only after the use of ozone therapy as an adjuvant therapy was tissue damage fully recovered, and the patient reported no pain; he could stop antibiotic, antimycotic, analgesic and opioid treatment and start chemotherapy again.
The results of this clinical case are highly encouraging, although further research is needed to standardize the ozone therapy clinical procedure and its indications with large sample sizes and prospective studies.