ABSTRACT
Fasciitis with eosinophilia (FE) is a rare connective tissue disease. Due to its rarity, large-scale studies are lacking, which makes its treatment challenging. Systemic corticosteroids (SCSs) are the cornerstone of treatment; however, additional immunosuppressive drugs (ISDs) are frequently necessary (usually methotrexate). We report 2 patients, for whom an SCS and methotrexate were not a viable long-term option. In the first case, we were unable to taper the SCS dose without symptom relapse, the patient showed only a partial response to methotrexate and presented side effects. The second case never fully responded to the SCS and methotrexate and demonstrated serious SCS adverse effects. Both patients were started on tocilizumab with extremely favourable results, making this drug a potential therapeutic weapon for these patients.
LEARNING POINTS
- The treatment of FE is challenging and mainly based on retrospective reviews, open-label trials and case reports, all of which included a small number of patients.
- Currently, systemic corticosteroids are the mainstay of treatment; however, other ISDs are frequently necessary.
- Cases showing a favourable clinical response to tocilizumab have recently been described in patients with corticosteroid-refractory disease, suggesting that this drug may potentially become a therapeutic weapon for these patients.
KEYWORDS
Eosinophilic fasciitis, Shulman's disease, tocilizumab
INTRODUCTION
Fasciitis with eosinophilia (FE) is a rare connective tissue disease characterized by symmetrical and painful swelling, progressive induration and thickening of the skin and subcutaneous tissue of distal extremities. A "peau d'orange" appearance (Fig. 1) and a "groove sign" (Fig. 2) are specific characteristics of this disease. Universally accepted diagnostic criteria are lacking, and thus, the diagnosis is based on clinical and laboratory characteristics (not mandatory), magnetic resonance imaging and a skin biopsy [1, 2]. Systemic corticosteroids (SCSs) are the mainstay of treatment. However, most studies suggest that additional immunosuppressive drugs (ISDs) are frequently necessary [2, 3].
CASE DESCRIPTION
Case 1
A previously healthy 37-year-old male presented with a 2-year history of skin erythema and thickening affecting the limbs and lumbar region, and sparing the skin of the face, hands and feet. He also complained of pain in the lower limbs and joint stiffness in the knees, ankles and wrists, precluding him from working. Furthermore, he reported fatigue and weight loss. These symptoms improved during a previous 3-month cycle of an SCS (prednisolone 60 mg/day), but relapsed after its suspension. Physical examination showed diffuse cutaneous cyanosis and indurated skin, especially in the forearms and legs but sparing the fingers, with a "peau d’orange" appearance involving the proximal areas and a "groove sign" visible on both forearms. Laboratory tests, including for peripheral eosinophils, the erythrocyte sedimentation rate (ESR) and immunological studies (immunoglobulins, antinuclear, antineutrophil cytoplasmic, anticentromere, anti-Scl-70, anti-U1 RNP and anti-RNA polymerase III antibodies), were all within the normal range. Work-up for HIV, hepatotropic viruses and latent tuberculosis was negative. The patient was submitted to thoracic radiography and oesophageal manometry, both of which showed normal results. Solid neoplasms were excluded. He had had a previous full thickness incisional skin biopsy, carried out after the corticosteroid cycle, that showed some characteristics suggesting FE: perivascular inflammatory cell infiltrate involving the fascia and adipose tissue as well as fascia and fibrous septae thickening. Considering the typical clinical presentation and skin biopsy, the diagnosis of FE was made, and the patient was started on an SCS (prednisolone 20 mg/day) and methotrexate (MTX; 12.5 mg/week). He responded favourably but with corticosteroid tapering and then suspension, symptoms relapsed. Hence, the SCS was reintroduced (prednisolone 5 mg/day) and the MTX dose was up-titrated (20 mg/week). However, due to hepatic toxicity and a concomitant insufficient response, MTX was discontinued. The patient was then started on tocilizumab (162 mg/week), with an excellent and sustained clinical response: after 1 year of treatment, the corticosteroid was successfully suspended. At the time of writing, the patient has been under tocilizumab alone for the last 12 months, showing significant and progressive improvement of the skin thickening (especially in the limbs) and absence of skin erythema, cyanosis, limb pain, joint stiffness or fatigue. He recovered his weight loss and is now able to work.
Case 2
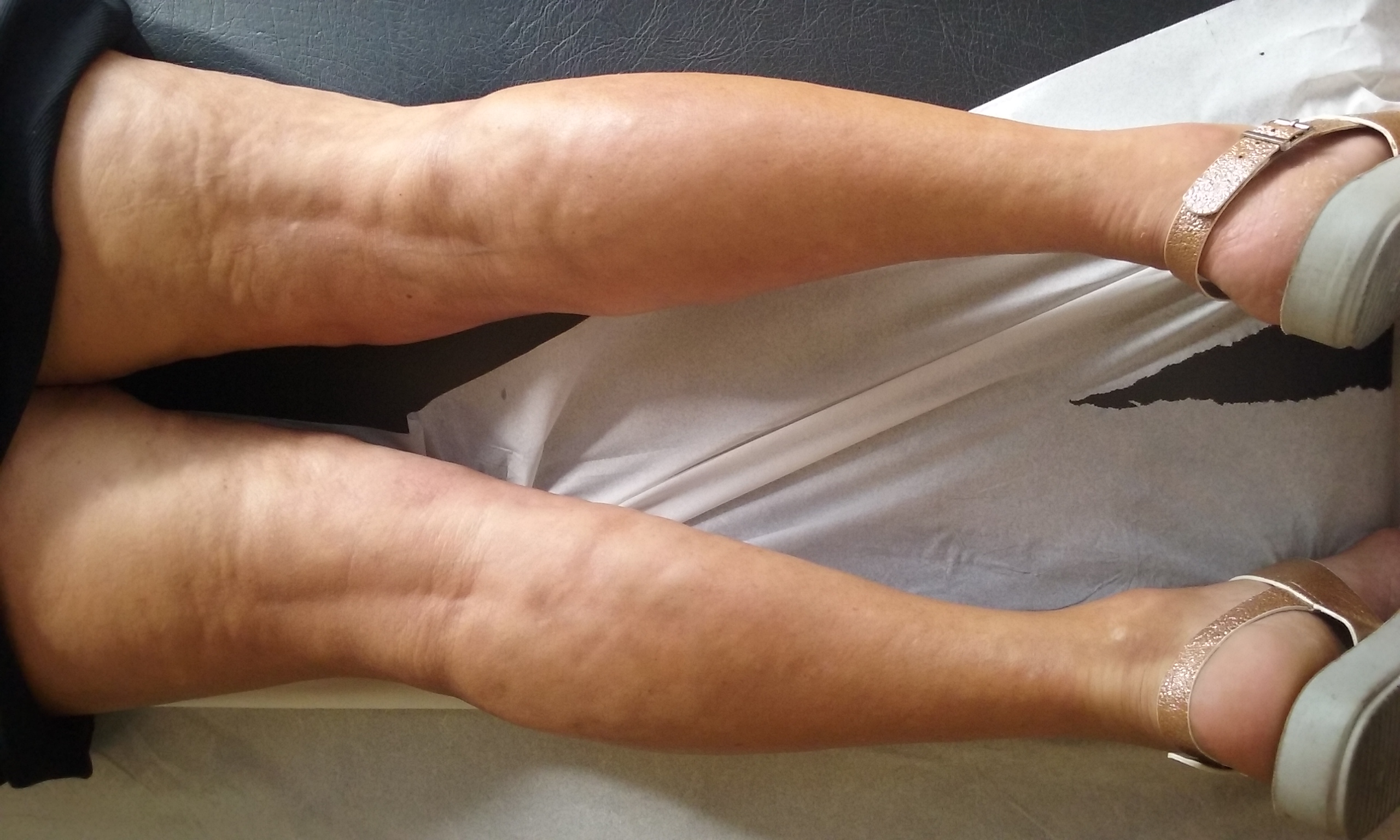
A 61-year-old female, with a history of diabetes mellitus, presented with a 2-year history of skin thickening affecting the lower limbs, inner thighs, lumbar region, abdominal flanks and forearms. She also reported asthenia and arthralgia (tibiotarsal joint). She denied having dysphagia or Raynaud's phenomenon. Physical examination showed indurated skin in the regions previously mentioned, sparing the fingers. These areas were hyperpigmented. She also had a "peau d’orange" appearance involving both legs (Fig. 1) and forearms as well as a "groove sign" visible on both forearms (Fig. 2).
Figure 1. "Peau d’orange" appearance involving both legs
Figure 2. "Groove sign" visible on the left forearm
Laboratory tests showed peripheral eosinophilia and an elevated ESR. Much like the previous patient, solid neoplasms were excluded, immunological studies were within the normal range and work-up for HIV, hepatotropic viruses and latent tuberculosis was negative. Pulmonary function tests and the thoracic CT scan were all normal. A full thickness skin biopsy showed fibrosis of the dermis, subcutaneous adipose tissue, fascia and muscle, with perivascular lymphocytes and eosinophil infiltration compatible with FE. The diagnosis of FE was made, and the patient was started on an SCS (prednisolone 60 mg/day) and MTX (15 mg/week), resulting in partial improvement of the lesions: lumbar region and forearms. However, due to evidence of corticosteroid adverse effects, such as uncontrolled diabetes and femoral head avascular necrosis, the dose was slowly tapered, while MTX was raised (until it was 20 mg/week). Considering that after 9 months of treatment she only demonstrated a partial response to MTX (20 mg/week), we decided to start the patient on tocilizumab (8 mg/kg). After 2 cycles of tocilizumab she finally started to show leg improvement, regaining mobility and enabling us to suspend MTX. At the time of writing, the patient is under tocilizumab alone and maintains clinical improvement: skin thickening limited to distal lower limbs.
DISCUSSION
FE was first described in 1974 by Shulman, and since then, only a few hundred cases have been reported[1, 2]. Due to its rarity, large-scale studies are lacking[4]. Therefore, treatment is challenging and mainly based on retrospective reviews, open-label trials and case reports, all of which have included a small number of patients. Furthermore, assessment of the clinical response is difficult due to the frequent concurrent use of SCSs, the lack of uniform outcome measures and the known natural history of this disease[3]. Currently, SCSs are the mainstay of treatment. However, additional ISDs are frequently required due to an unsatisfactory clinical response or as corticosteroid-sparing agents[2,3]. The most commonly used is MTX with studies increasingly favouring the combination of this ISD with SCSs as the initial treatment of choice[3]. Other treatments have been described but with anecdotal benefits. These include azathioprine, hydroxychloroquine, sulfasalazine, rituximab, infliximab, among others[4]. In 2015, a patient with corticosteroid-refractory disease and no response to MTX or etanercept was, for the first time, successfully treated (off-label) with tocilizumab[5]. Since then, a few similar case reports have been described[4]. Tocilizumab is a humanized monoclonal antibody against the interleukin-6 (IL-6) receptor, which has demonstrated efficacy and safety in the treatment of rheumatoid arthritis. It binds to IL-6 receptor α and inhibits IL-6-mediated pro-inflammatory signalling. Considering that IL-6 seems to stimulate collagen production and is implicated in fibrosis development, an IL-6 inhibitor such as tocilizumab could theoretically be effective in FE[4]. We present 2 patients in whom this ISD was successfully used. In the first case, although the patient responded favourably to the SCS, we were unable to taper the dose without symptom relapse. On the other hand, MTX only had a partial effect on the disease and was associated with adverse effects. The second patient never fully responded to the SCS or MTX and started demonstrating serious corticosteroid side effects. Both patients were started on tocilizumab, which was very well tolerated and allowed us to suspend MTX and the SCS, with extremely favourable results.
In conclusion, FE is a rare but highly incapacitating disease that is challenging to treat. Although SCSs remain the cornerstone of treatment, other ISDs are starting to be considered in association or alone to maximize the clinical response or as corticosteroid-sparing agents. Although current evidence is still limited to a few case reports, tocilizumab seems to provide an effective and sustained response and may potentially become a therapeutic weapon for these patients. Further studies, ideally with larger population samples, are needed[4].