ABSTRACT
We describe a rare presentation of acute pyelonephritis associated with a ruptured abdominal aortic aneurysm. A 68-year-old female presented to the emergency department with a 3 day history of cystitis. General examination revealed the acute onset of pain in the left flank accompanied by fever and chills. Blood tests revealed leucocytosis 25,400x109L and C-reactive protein 495 mg/L (<6.1), while urinary sediment analysis revealed many leucocytes and gram-negative bacteria. The patient was admitted with acute pyelonephritis. On the third day of admission, the urine culture isolated Escherichia coli sensitive to the antibiotic prescribed; however, the patient clinically deteriorated. A computed tomography scan revealed a ruptured abdominal aortic aneurysm involving the left renal artery. The patient underwent an exploratory laparotomy but uncontrollable haemorrhage led to a fatal outcome.
This case highlights a rare case of acute pyelonephritis associated with a ruptured abdominal aortic aneurysm. A computed tomography scan or abdominal ultrasound should be considered whenever a patient has acute pyelonephritis with a C-reactive protein >400 mg/L in order to exclude complications and other potentially fatal pathologies.
LEARNING POINTS
- Acute pyelonephritis can lead to a ruptured abdominal aortic aneurysm.
- There should be a high index of suspicion for other concomitant acute pathologies in patients with pyelonephritis and a C-reactive protein >400 mg/L.
- A low threshold for abdominal imaging, either a computed tomography scan or abdominal ultrasound, may allow for the diagnosis of pathologies with a high mortality rate, such as a ruptured abdominal aortic aneurysm, at an early stage and thus result in better prognosis.
KEYWORDS
Acute pyelonephritis, ruptured abdominal aortic aneurysm, abdominal ultrasound, C-reactive protein
CASE DESCRIPTION
A 68-year-old female was asymptomatic until three days before admission to the emergency department, when she developed symptoms suggestive of cystitis (dysuria, frequency, haematuria). Other than a depressive disorder and type 2 diabetes, her past medical history was unremarkable. General examination on admission revealed the acute onset of pain in the left flank accompanied by fever and chills. Blood tests revealed normocytic normochromic anaemia with Hb 11.1 g/dL, leucocytes 25,400x109/L, thrombocytosis with a platelet count of 473,000x109/L, blood urea nitrogen 34 mg/dL, creatinine 0.86 mg/dL, and C-reactive protein (CRP) 495 mg/L (<6.1). Examination of the urinary sediment revealed many leucocytes and gram-negative bacteria as well as occasional pyocytes and erythrocytes. The patient was commenced on cefuroxime and admitted to the internal medicine department with acute pyelonephritis.
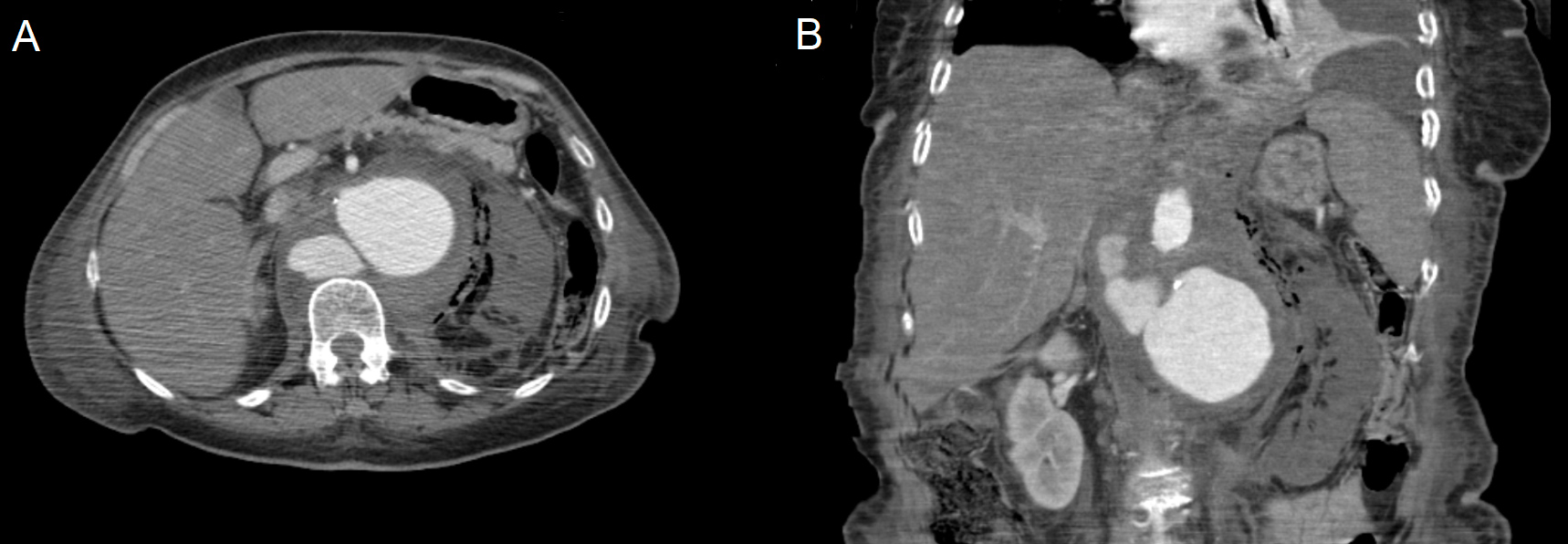
On the third day of admission, the urine culture revealed Escherichia coli sensitive to the previously prescribed cefuroxime, but with no signs of clinical improvement. The patient was pale, confused, tachypnoeic, tachycardic and hypotensive, with a systolic pressure of 78 mmHg. She was unresponsive to fluids and her abdomen was tender on the left flank with no peritoneal signs. Blood analysis showed that her haemoglobin level had dropped to 8.2 g/dL, leucocytes increased to 33,400x109/L, and CRP was 398 mg/L, which led to a suspicion of septic shock secondary to complicated pyelonephritis. An abdominal and pelvic computed tomography (CT) scan was performed, which revealed an abdominal aortic aneurysm (AAA) arising below the coeliac trunk with a hyperdense retroperitoneal haematoma. The aneurysm involved the left renal artery with no contrast enhancement in the left kidney but with gas accumulation in the pararenal space (Fig. 1). The maximum diameter of the AAA sac was 72 mm; there were also two other sacs with diameters of 40 mm and 31 mm, corresponding to organised haemorrhagic leaks, in relation to this aneurysm and posterior to the inferior vena cava.
Figure 1. CT images (A and B) after intravenous iodinated contrast showing an abdominal aortic aneurysm with two other sacs corresponding to organised haemorrhagic leaks and a left kidney with no contrast enhancement
The patient was urgently referred to the vascular surgery department and underwent an exploratory laparotomy. However, the rupture was uncontrollable and she died following cardiac arrest secondary to hypovolaemic shock.
DISCUSSION
Making a diagnosis of ruptured AAA in the emergency department is a clinical challenge, more so when other pathologies coexist. Clinical features of a contained AAA rupture are vague, with back pain in 64% of cases, abdominal pain in 20%, groin pain in 14%, sepsis in 10%, femoral neuropathy in 8%, and asymptomatic in 8%[1].
At first glance, when the patient showed clinical deterioration despite adequate antibiotic treatment, we assumed that she had a complicated pyelonephritis. The CT scan to rule out renal or perinephric abscess formation, emphysematous pyelonephritis, renal vein thrombosis or papillary necrosis, surprisingly showed a leaking AAA. The triad of acute abdominal or lumbar pain, hypotension and shock is the most usual presentation in ruptured AAA[2,3], but in our case this did not occur. Our case provides a clinical challenge because, on the one hand, we had a ruptured AAA with a contained haematoma masking the extent of the symptoms and, on the other hand, history, symptoms and blood analysis suggestive of pyelonephritis as well as a positive urine culture for Escherichia coli, which is usually present in urinary tract infections.
In our opinion, it would appear to be a case of aortic aneurysm rupture occurring after acute pyelonephritis and not of aortic aneurysm rupture initially misdiagnosed as acute pyelonephritis, given that the decrease in haemoglobin levels only occurred on the third day post-admission and hitherto had been stable. One mechanism for the rupture is that in the AAA wall there is upregulation of pro-inflammatory cytokines, IL-1β, IL-6, IL-10 and TNF-α, which have been shown to positively correlate with aneurysm growth. During septic events, in this case due to acute pyelonephritis, such cytokines, chemokines and growth factors are known to be potentiated[4, 5], which eventually may have led to the rupture.
The AAA involved the left renal artery and the left kidney therefore had no contrast enhancement; there was also gas accumulation in the pararenal space most likely secondary to infection and renal necrosis.
Studies show that mean values of CRP in upper urinary tract infection (UUTI) were around 157 mg/L with a standard deviation of 86 mg/L[6,7]; other studies show that CRP protein increases in ruptured AAA ranging between 6.5 mg/L and 85 mg/L[8]. In our opinion, a CRP higher than what is expected for a UUTI may reflect a complication or other concomitant pathologies like the ruptured AAA. Hence, we recommend that physicians order a CT scan or abdominal ultrasound when CRP is higher than 400 mg/L in patients presenting with acute pyelonephritis.
According to recent studies, AAA screening programmes are effective in detecting[9,10] and treating AAA[11]. We believe our case strengthens the argument for performing an abdominal ultrasound as it is a feasible tool, reproducible and safe, without risk of radiation.
We urge physicians to consider abdominal imaging, either a CT scan or abdominal ultrasound, whenever a patient presents with pyelonephritis and a C-reactive protein >400 mg/L in order to exclude pyelonephritis complications or other acute pathologies such as AAA. Pathologies with a high mortality rate may therefore be diagnosed at an early stage resulting in better prognosis.