ABSTRACT
Interrupted aortic arch (IAA) is an extremely rare congenital cyanotic heart disease characterized by complete disruption between the ascending and descending aorta. A patent ductus arteriosus (PDA) or other collateral pathways provide blood flow to the distal descending aorta. Mortality is extremely high at early infancy, particularly after closure of the ductus arteriosus. Survival and presentation in adulthood are extremely rare. Here, we illustrate a rare case of type B IAA in an adult who presented with secondary polycythaemia. The blood supply to the descending aorta and beyond was almost solely via a PDA. This case demonstrates the value of multimodality imaging, including CT and MRI, for diagnosis and treatment planning in these patients.
LEARNING POINTS
- The presence of secondary polycythaemia, as a result of chronic hypoxia, should prompt a search for underlying cyanotic heart disease even in previously undiagnosed adults.
- Most previous case reports of IAA in adults feature type A; type B IAA in an adult is far less frequently described.
- MRI has incremental value compared to CT in intracardiac assessment (aortic valve assessment, RV and LV functional assessment, flow measurement) for these patients; in addition, it provides an excellent depiction of the vascular anatomy of the aorta and great vessels./li>
KEYWORDS
Interrupted aortic arch, congenital heart disease, secondary polycythaemia, computed tomography, magnetic resonance imaging
CASE DESCRIPTION
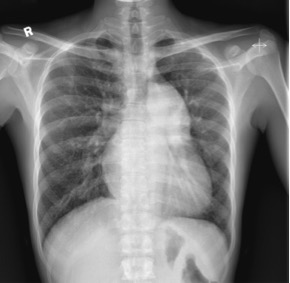
A 40-year-old man presented to the medical unit with worsening dizziness and reduced exercise tolerance over the previous few months. He reported 1 episode of syncope 2 years earlier. There were no symptoms of lower limb claudication. He was a non-smoker and non-drinker with no family history of cardiac disease. On physical examination, he was mildly hypertensive with blood pressure 159/86 mmHg. He was tachypnoeic with oxygen saturation (SaO2) of 87% on room air. Central cyanosis and finger clubbing were present. A 12-lead electrocardiogram (ECG) showed partial right bundle branch block (RBBB) with features of right ventricular hypertrophy. A 6-minute walking test for exercise tolerance showed desaturation from 83% to 63% after 6 minutes. Chest radiography (Fig. 1) showed gross cardiomegaly and a dilated pulmonary trunk.
Routine blood tests showed a haemoglobin (Hb) level of 22.0 and a haematocrit (Hct) of 0.70. Erythropoietin (EPO) levels were increased, suggestive of secondary polycythaemia rather than polycythaemia rubra vera. There was mild thrombocytopenia with a platelet count of 56×109/l.
In view of the presence of polycythaemia, the initial clinical suspicion was that of lymphoproliferative disease, for example, lymphoma, or an EPO-producing tumour with paraneoplastic syndrome. Therefore, contrast-enhanced computed tomography (CT) was arranged.
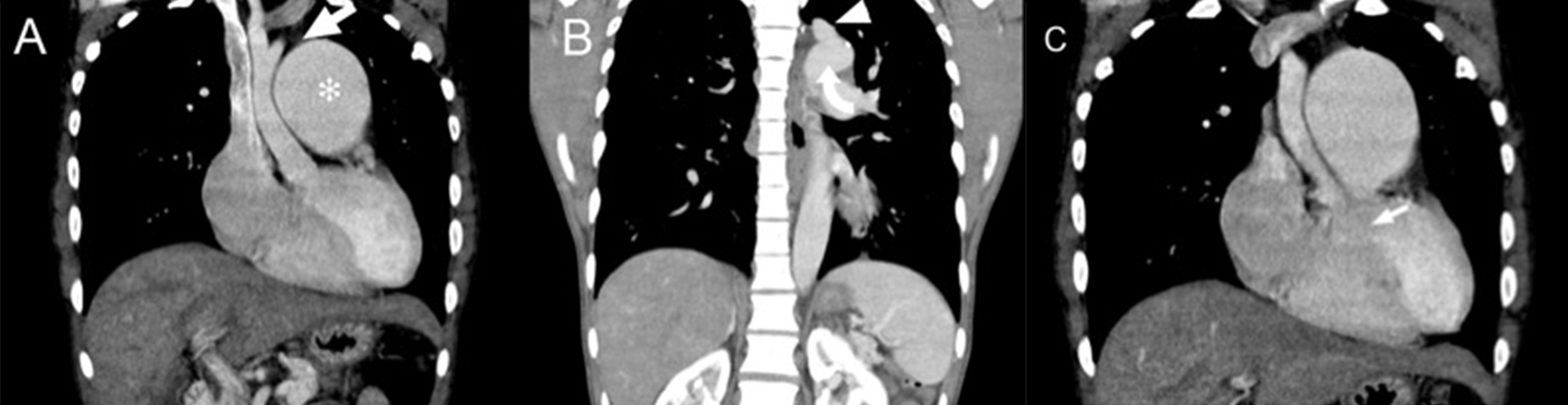
CT (Fig. 2) revealed discontinuity between the ascending aorta and descending aorta, with the descending aorta and left subclavian artery supplied by the grossly enlarged pulmonary trunk (measuring 6 cm in diameter) via a patent ductus arteriosus (PDA). The ascending aorta was of small calibre. These findings were consistent with type B interrupted aortic arch (IAA).
Figure 1. The chest radiograph showed a grossly enlarged cardiac shadow, an enlarged pulmonary trunk and a small-calibre ascending aorta. No rib notching was noted Figure 2. Computed tomography images showing: (A) and (B) a small-calibre ascending aorta with the aortic arch interrupted after the origin of the left common carotid artery (arrow in A) and separated from the left subclavian artery (arrowhead in B) and descending aorta (curved arrow in B). The pulmonary trunk (* in A) was markedly dilated. (C) A ventricular septal defect could also be visualized (arrow)
A transthoracic echocardiogram was obtained and showed evidence of right atrial enlargement and right ventricular hypertrophy. A ventricular septal defect (VSD) measuring 2.2 cm × 1.2 cm with a predominant left-to-right shunt was seen. A tubular structure (1.8 cm in diameter) was seen connecting the main pulmonary artery to the descending aorta, consistent with a large PDA.
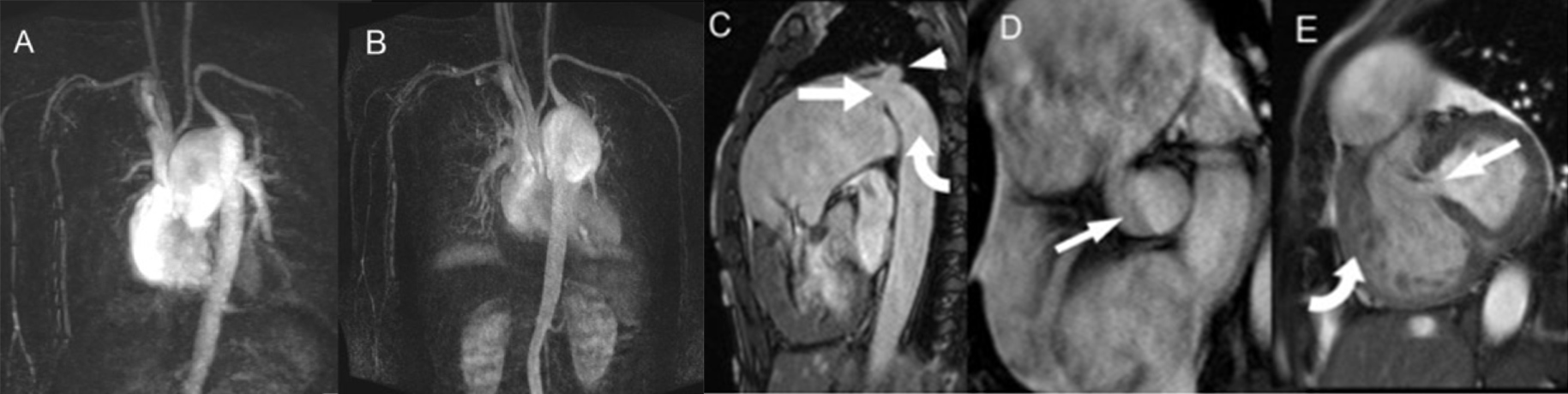
Magnetic resonance imaging (MRI) with MR angiography (MRA) was also performed (Fig. 3). In addition to visualization of the IAA, dilated pulmonary trunk, PDA and VSD, phase contrast imaging also demonstrated large forward flow in the pulmonary artery of 91 ml with a reverse flow of 22 ml. To-and-fro flow was also seen in the PDA. The forward flow at the ascending aorta was only 15 ml. This was suggestive of a haemodynamically significant shunt. Cardiac MRI also revealed a mildly impaired right ventricular ejection fraction (47%) and normal left ventricular function (52%). MRA showed no significant collateral flow from the intercostal arteries or internal mammary arteries.
Venesection was performed to relieve symptoms related to polycythaemia. The patient’s Hb levels were lowered with subjective improvement in symptoms. Combined cardiac and cardiothoracic surgical assessment was planned for this patient for discussion of possible cardiac surgery but the patient was subsequently lost to follow-up.
Figure 3. Magnetic resonance imaging (MRI). (A) and (B) Contrast-enhanced magnetic resonance angiography (MRA) with subtraction showing aortic interruption and an enlarged pulmonary artery supplying the left subclavian artery and descending aorta via a patent ductus arteriosus. No significant collateral flow was present. (C) T2W TRUFI sagittal image showing a patent ductus arteriosus (straight arrow) supplying the left subclavian artery (arrowhead) and descending aorta (curved arrow). (D) SSFP cine image showing a bicuspid aortic valve (arrow). (E) Evidence of a ventricular septal defect (arrow) and right ventricular hypertrophy (curved arrow) was also seen
DISCUSSION
Cyanotic congenital heart disease is usually recognized as a cause of polycythaemia in infants/children with right-to-left shunts. In the adult population with no known underlying cardiac disease, there is often less awareness of this as a cause of polycythaemia. This is illustrated in our patient with an ‘incidental’ detection of IAA, when CT was performed for investigation of polycythaemia with the suspicion of underlying lymphoma or paraneoplastic syndrome. In our case, polycythaemia was secondary to chronic hypoxia as a physiological response[1]. To the best of our knowledge, there have been no previous case reports documenting polycythaemia as a presenting abnormality in patients with IAA.
Three types of IAA are described[2], depending on the site of interruption. Type A (interruption distal to the origin of the left subclavian artery) is most common in adults, while type B (interruption between the left common carotid artery and the left subclavian artery) is common in infants but much rarer in adults. The majority of previous adult case reports feature type A, while type B IAA in adults is less well reported[3]. In a previous review of 38 cases of IAA in adults by Gordon et al.[4], only 6 of these (16%) were of type B. All types of IAA are associated with other aortic or cardiac abnormalities, including the presence of a bicuspid aortic valve, a PDA, a VSD, subaortic stenosis, transposition of the great arteries (TGA) and anomalous subclavian arteries[5,6].
>
In patients with IAA, survival depends on collaterals to supply the descending aorta and beyond. There is usually formation of collaterals as the PDA closes gradually. However, in our case, collaterals were minimal as the PDA remained large all along with significant supply to the descending aorta and left subclavian artery, as demonstrated by the flow information obtained from MRI. In a review by Sharratt et al.[7], of the 9 cases that survived beyond childhood, only 2 had a persistent ductus arteriosus. In our patient, the ductus arteriosus did not close and provided adequate blood flow to the descending aorta to prevent distal ischaemia and limb claudication symptoms, although evidence of significant pulmonary hypertension was already present and should be managed.
A multimodality imaging approach is crucial for the diagnosis and management of IAA. CT provides an excellent view of the vascular anatomy and presence of collaterals but has ionizing radiation, which is a particular concern if imaging follow-ups are considered. It provides no functional assessment and very limited intracardiac assessment. An echocardiogram is unlikely to visualize the interrupted arch itself but is useful for assessment of associated cardiac and aortic abnormalities, including intracardiac septal defects, a bicuspid aortic valve, as well as functional assessment of the left ventricle. Its usefulness may, however, be impaired by body habitus or a limited acoustic window. Echocardiography is usually not optimal for right heart assessment. MRI, on the other hand, can reveal both functional and anatomical details in IAA and its associations, combining the functional information from an echocardiogram with the anatomical details of CT[8].
The management of secondary polycythaemia is controversial, with doubtful benefits of venesection[9]. Surgical management is the mainstay in cases of IAA in adults[4,7]. As our patient was lost to follow-up, the eventual outcome is uncertain.