HOSPITAL GRAND ROUNDS
UKC Maribor, by Editorial Board Member Radovan Hojs
University Medical Centre (UKC) Maribor, Slovenia, provides secondary and tertiary healthcare services to patients from Maribor and northeastern part of Slovenia. It has status of teaching hospital and it is learning base for medical students. The origin of UKC Maribor reaches back to the Maribor city hospital that has been treating patients since 14th century and constantly developing since its official establishment in 1799. Today UKC Maribor employs 3400 staff members, of them approx. 600 medical doctors. Part of UKC Maribor is Clinic for Internal medicine that includes departments of Cardiology and Angiology, Gastroenterology, Endocrinology and Diabetology, Nephrology, Dialysis, Rheumatology, Haematology and Hematologic Oncology, Pulmonary Diseases, Nuclear Medicine, Internal Intensive Medicine and Emergency Medical Unit.
ABSTRACT
Amiodarone is an antiarrhythmic drug, in use from the 1960s, which acts on potassium transport in myocytes, causing a lengthening of the action potential and refractory period. Even though it is broadly prescribed, its use is limited by a relatively high occurrence of adverse reactions such as lung, thyroid or hepatic disease, skin changes and so on. The authors report a case of a female patient who was admitted due to chest pain. Due to the bluish skin pigmentation, other causes of amiodarone toxicity were investigated, and hyperthyroidism was detected. After amiodarone discontinuation and specific therapy, thyroid function returned to normal.
LEARNING POINTS
- Blue pigmentation of facial skin is an uncommon adverse effect of chronic amiodarone therapy that occurs in less than 3% of patients.
- When a patient is on chronic amiodarone therapy, signs of toxicity, such as hyperthyroidism, lung injury or hepatic disease, should be investigated.
- Regular liver and thyroid function tests and chest x-rays should be carried out on follow-up after initiation of amiodarone.
KEYWORDS
Amiodarone, adverse drug reaction, hyperthyroidism, atrial fibrillation, skin pigmentation
INTRODUCTION
Amiodarone is a commonly used antiarrhythmic drug, mostly for treatment of ventricular arrhythmias and symptomatic atrial fibrillation[1]. It has a high iodine content (37% by weight) and acts pharmacologically to inhibit the monodeiodination of thyroxine (T4), which often results in changes in thyroid function tests[2]. Furthermore, it is associated with thyroid dysfunction that can present itself as amiodarone-induced hypothyroidism (AIH), present in approximately 5% of amiodarone-treated patients, or thyrotoxicosis (AIT), present in 7–15% of amiodarone-treated patients[2,3]. AIT can be further classified into type 1, type 2 or mixed type. Type 1 is a form of iodine-induced hyperthyroidism that usually develops in nodular goitre or latent Graves’ disease, type 2 is a destructive thyroiditis occurring in an otherwise healthy thyroid, while a mixed/indefinite type presents an overlapping condition[2].
The use of amiodarone is limited by its toxicity presenting as hepatic, gastrointestinal, pulmonary, thyroid, neurologic, dermatologic, ophthalmologic, haematologic, psychiatric or cardiac adverse effects[4]. Although uncommon, bluish skin pigmentation is present in 1–7% of patients on chronic amiodarone therapy, while photosensitivity appears in 24–57%. This is thought to be due to lipofuscin accumulation in lysosomes in sun-exposed areas due to phototoxic damage or drug deposition within dermal cells. Other adverse effects due to amiodarone have no known association to bluish skin discoloration[4,5]. Bluish skin discoloration has also been described in several conditions such as argyria, methaemoglobinaemia and ochronosis, and has been associated with several medications such as amiodarone, minocycline, chlorpromazine, chloroquine and hydroxychloroquine[6].
CASE DESCRIPTION
An 84-year-old female was admitted to the Internal Clinic for further work-up of a possible non-ST elevation myocardial infarction (NSTEMI). She had previously known chronic atrial fibrillation, arterial hypertension, diabetes mellitus type 2, and aortic and mitral insufficiency. On the day of admission, she felt an intense, sharp pain in her back that radiated to the left side of her thorax. Additionally, she described the pressure on her chest as stenocardic, only mildly alleviated by analgesics. Her regular therapy consisted of atorvastatin, valsartan, hydrochlorothiazide and amiodarone.
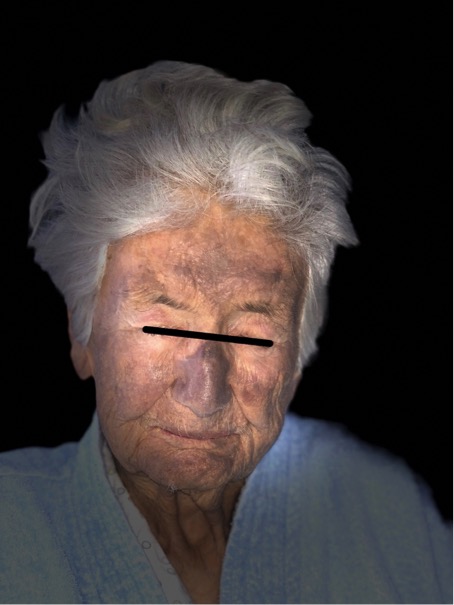
At admission she was oriented, afebrile, her blood pressure was 180/65 mmHg, heart rate 63 beats/min, peripheral oxygen saturation (SpO2) 99%. The only abnormal findings in the clinical examination were bluish skin pigmentation of the face (Fig. 1), which the admitting doctor suggested was due to amiodarone, a systolic murmur and irregular heartbeat.
The ECG showed left axis deviation, sinus rhythm with a ventricular frequency of 65 beats/min, ST depression in D1, aVL, V4–5, biphasic T waves in V2–5 and signs of left ventricular hypertrophy. The ECG changes were not present in the previous ECG carried out 6 months prior.
The initial laboratory investigation showed normocytic anaemia (haemoglobin 112 g/l), hypokalaemia (2.7 mmol/l) and low vitamin B12 (125 pmol/l). Serial troponin measurements (5x) were negative.
During the patient’s hospitalization, chest x-ray showed dilatation of mediastinal vasculature and echocardiography showed dilatation of the ascending aorta. Thoracic computed tomography angiography was carried out, which showed a dilatation of the ascending aorta and changes in the descending aorta that could represent multiple penetrating ulcers evolving into an intramural haematoma and dissection with possible rupture. Cardiac surgeons and intervention radiologists were consulted and suggested conservative therapy for chronic type B dissection.
Due to the bluish skin pigmentation and history of chronic amiodarone therapy, which the patient received for atrial fibrillation for approximately 8 years, according to the hospital medical record, thyroid function tests were performed. Initial thyroid function tests showed: thyroid-stimulating hormone (TSH) 0.369 mU/l (reference values 0.27–24.2 mU/l), free thyroxine (FT4) 28.81 pmol/l (reference values 12.0–22.0 pmol/l), free triiodothyronine (FT3) 2.65 pmol/l (reference values 3.1–6.8 pmol/l). A thyroid ultrasound showed normal thyroid size, somewhat inhomogeneous tissue and several nodules with cystic degeneration, the largest measuring 9 mm. Testing for thyroid antibodies (antithyroid peroxidase antibody, aTPO; thyroid-stimulating immunoglobulin, TSI; antithyroglobulin antibody, aTg) was negative. Amiodarone was discontinued and thiamazole 10 mg twice daily was prescribed for AIT. A thyroid function test check-up after 2 weeks showed: TSH 0.055 mU/l, FT4 30.97 pmol/l, FT3 2.24 pmol/l. Thiamazole was elevated to 10 mg 3 times daily, while sodium perchlorate 3 × 15 drops for 3 weeks and methylprednisolone 24 mg were additionally prescribed. One month later, the follow-up thyroid function tests showed: TSH 1.15 mU/l, FT4 18.88 pmol/l, FT3 1.4 pmol/l. Thiamazole was lowered to 5 mg 3 times daily and methylprednisolone was tapered 8 mg per week until discontinuation. The last thyroid function test check-up 1.5 months later showed: TSH 14.73 mU/l, FT4 12.55 pmol/l, FT3 3.06 pmol/l. Thiamazole was discontinued.
DISCUSSION
Patients that have photosensitivity or have developed bluish skin discolouration due to amiodarone treatment are advised to avoid sun exposure, use sun-protective clothing and apply sunscreen. Cases have shown resolution of discolouration typically 1 year after discontinuation of amiodarone[4].
In patients on chronic amiodarone therapy, regular follow-up is necessary. In addition to patient history and examination, baseline liver and thyroid function tests, chest x-ray, pulmonary function tests and electrocardiographic assessment should be conducted. Liver and thyroid function tests should be repeated every 6 months; chest x-ray every year[1].
The European Thyroid Association (ETA) published new guidelines regarding amiodarone-associated thyroid dysfunction in 2018. For AIT they recommend emergency treatment, especially in elderly patients or if left ventricular dysfunction is present. Discontinuation of amiodarone therapy is dependent on the underlying arrhythmia and AIT type. For AIT type 1, antithyroid drugs should be used along with sodium perchlorate to accelerate control of hyperthyroidism. For AIT type 2, oral glucocorticoids are considered first-line therapy. For mixed type AIT, thionamides should be given initially with the addition of glucocorticoids with a poor response. The ideal timing for glucocorticoid therapy for mixed type AIT is still unknown[2]. For AIT type 1 patients who need on-going amiodarone therapy, thyroidectomy is recommended once AIT is under control[3]. Our patient was started on thiamazole with the addition of sodium perchlorate and methylprednisolone because of a lack of response. With combined therapy she became euthyroid.