ABSTRACT
Reactivation of human parvovirus B19 is exceptional and characteristic of immunosuppression, with anaemia being the predominant manifestation although pancytopenia and thrombotic microangiopathy may also occur. We describe a patient with a history of diffuse large B-cell lymphoma with pure erythrocyte aplasia due to reactivation of parvovirus B19, who was treated with corticosteroids and immunoglobulins.
LEARNING POINTS
- Infection with human parvovirus B19 is identified by polymerase chain reaction (PCR) testing of blood and the presence of typical giant proerythroblasts in the bone marrow.
- Cytomegalovirus infection should be considered in immunosuppressed patients with fever and non-specific symptoms with haematological changes.
- The treatment of persistent infection in immunosuppressed patients is based on the administration of IV immunoglobulins at high doses.
KEYWORDS
Human parvovirus B19, pure red cell aplasia, viral reactivation
CASE DESCRIPTION
A 62-year-old patient presented with a history of heart failure with depleted left ventricular ejection fraction and diffuse large B-cell lymphoma (non-germinal phenotype, Ann Arbor stage IIB). The lymphoma had been treated 2 years previously with chemotherapy consisting of rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) and second-line therapy with prednisone/rituximab plus bendamustine and splenectomy, and the patient had achieved complete remission. Rituximab had been prescribed for maintenance therapy since then. The patient was admitted to the internal medicine department due to decompensated heart failure and febrile syndrome with no apparent focus. Empirical broad-spectrum antibiotics were administered without improvement. Serial blood cultures, urine cultures and urine tests for pneumococcus and legionella were all negative. It was suggested that the illness might be due to recurrence of lymphoma.
During hospitalization, the patient’s cardiorespiratory symptoms improved but warm antibody haemolytic anaemia was detected (haemoglobin level of 7 g/dl with a mean corpuscular volume of 93 fl, and a positive Coombs test). Treatment for 7 days with methylprednisolone pulses controlled the anaemia. Blood tests revealed a tendency to macrocytosis, while a bone marrow study excluded myelodysplastic syndrome. FISH was negative for myelodysplastic syndrome and cytomegalovirus activation was ruled out (DNA <150 copies/ml), but there was excessive replication of parvovirus B19 (DNA 4610 copies/ml) and signs in the bone marrow biopsy were compatible with the infection, which explains the crisis of pure red cell aplasia. We added intravenous immunoglobulins to the established treatment, at a dose of 0.4 mg/kg (total dose of 18 g), which resulted in improvement in haemoglobin levels.
DISCUSSION
Parvovirus B19 is the main pathogenic parvovirus in humans. It is a small virus approximately 20–25 nm in diameter, without a membrane covering, with icosahedral and capsid symmetry with two structural proteins. It belongs to the family Parvoviridae, genus Erythrovirus. It has a small single strand of DNA with 5.5 kilobases, with the correct information to replicate using enzymes produced during the S phase of the infected cell cycle. This last fact explains tropism by cells with active replication: it is predicted by the erythroid series, infecting and inhibiting the mitotic activity of erythroblasts. Infection of the cell is achieved through the P antigen (Gb4 globose), a receptor present in erythroid cells and others, including endothelial cells, platelets, synoviocytes, smooth muscle cells and fetal myocytes.
Anaemia is the clinical anomaly described most frequently in the context of parvovirus B19 infection [1]. Normocytic, severely normochromic, regenerative (with reticulopenia) anaemia does not respond to blood transfusions or erythropoiesis-stimulating agents. Thrombocytopenia and neutropenia are other blood abnormalities. Clinical manifestations such as fever and arthralgia may occur in infected patients [2]. Clinical manifestations of organ involvement such as hepatitis, myocarditis and pneumonitis can also be seen [1].
Although viral reactivation is uncommon, risk factors include congenital immunosuppression, HIV infection [3], leukaemia and lymphoma, the use of immunosuppressants [4] and, as in our case, the use of rituximab [5]. Other risks include solid organ transplantation [6], in particular kidney transplantation [1, 7–11] and allogeneic transplantation of haematopoietic precursors [12], which is infrequent but can occur during autologous transplantation [13]. At-risk patients include those with chronic haemolytic anaemia, either hereditary or acquired (sickle cell anaemia, thalassemia, hereditary spherocytosis, autoimmune haemolytic anaemia, pyruvate kinase deficiency, paroxysmal nocturnal haemoglobinuria, HEMPAS syndrome), iron deficiency anaemia, and acute loss of blood.
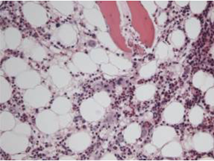
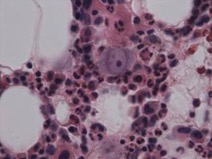
Parvovirus B19 infection was identified through PCR testing of blood and a bone marrow study which detected erythroid cells with signs of parvovirus B19 infection (Fig. 1), and pronormo-erythroblasts with nuclear viral inclusion characteristic of infection by parvovirus B19 (Fig. 2) [14]. The high viral load is associated with a more significant clinical course [15–17], although it is important to underline that the finding of low levels of DNAemia is not usually clinically important nor does it require therapeutic intervention [16, 17]. Low viral loads have also been detected in immunocompetent patients beyond the first 3 years of primary infection, with a possible cause being transfusion reaction [18].
The treatment of persistent infection in immunosuppressed patients is based on the administration of IV immunoglobulins (IVIG) at high doses: 400 mg/kg/day for 5 days or 1000 mg/kg/day for 2 days [19]. Vaccines are currently in the human trial phase. Our patient was successfully treated with IVIG without adverse events. Progressive increases in haemoglobin values were observed during the days after IVIG treatment.
Figure 1. Erythroid cells with signs of infection with parvovirus B19 (haematoxylin eosin, ×100)
Figure 2. Pronormo-erythroblasts with nuclear viral inclusion characteristic of infection by parvovirus B19 (haematoxylin eosin, ×400)
Current evidence does not support routine screening, except for research purposes. However, due to the serious clinical implications for immunosuppressed and transplanted patients, parvovirus B19 should be considered in patients with anaemia.
There is no general agreement on the usefulness of prophylaxis with immunoglobulins in patients with chronic haemolysis or immunodeficiency or in pregnant women at risk. A recombinant parvovirus B19 vaccine is currently being developed [20].