Interprofessional Management of Median Arcuate Ligament Syndrome (Dunbar Syndrome) Related to Lumbar Lordosis and Hip Dysplasia: A Patient’s Perspective
Sclinda Lea Janssen1, Thomas Scholbach
2, Susan Jeno
3, Holte Laurie
4, Mandy Meyer
1,5, Colin Combs
5
1 Department of Occupational Therapy, School of Medicine and Health Sciences, University of North Dakota, Grand Forks, ND, USA
2 Ultrasound Clinic, Leipzig, Germany
3 Department of Physical Therapy, School of Medicine and Health Sciences, University of North Dakota, Grand Forks, ND, USA
4 Achieve Therapy, Grand Forks, ND, USA
5 Department of Biomedical Sciences, School of Medicine and Health Sciences, University of North Dakota, Grand Forks, ND, USA
Doi: 10.12890/2020_001605 - European Journal of Case Reports in Internal Medicine - © EFIM 2020
Received:27/02/2020
Accepted: 11/03/2020
Published: 16/04/2020
How to cite this article: Janssen SL, Scholbach T, Jeno S, Laurie H, Meyer M, Combs C. Interprofessional management of median arcuate ligament syndrome (Dunbar syndrome) related to lumbar lordosis and hip dysplasia: a patient's perspective.
EJCRIM 2020;
7 doi:10.12890/2020_001605.
Conflicts of Interests: The authors declare there are no competing interests.
This article is licensed under a Commons Attribution Non-Commercial 4.0 License
ABSTRACT
We present a 53-year-old female patient with median arcuate ligament syndrome (MALS), also known as Dunbar syndrome or celiac artery compression syndrome, related to lumbar lordosis and hip dysplasia. She utilized interprofessional management strategies, which were beneficial in reducing lumbar lordosis and MALS-related symptoms. This finding is important because there are no other reports in the literature describing interprofessional strategies to manage symptoms for patients who are waiting for surgery or are not candidates for surgery.
LEARNING POINTS
- Excessive lumbar lordosis is related to the development of median arcuate ligament syndrome (MALS) due to the greater distance the median arcuate ligament stretches around the vertebral curves, causing compression of the celiac nerves and artery.
- It is important to consider the effects MALS has on multiple body systems when diagnosing and developing symptom management strategies.
- Referrals to interprofessional team members can help the patient manage the vast array of symptoms related to MALS.
KEYWORDS
Median arcuate ligament syndrome, lumbar lordosis, hip dysplasia, symptom management strategies, interprofessional
CASE DESCRIPTION
At age 51, the patient (the first author) consulted an internal medicine physician for multiple concerns: upper abdominal pain with eating, early satiation, 20-pound weight loss, epigastric pain, and upper body/head swelling, especially in her left temporal area, causing headaches. She had additional symptoms associated with multiple body systems including: dyspnoea, heart palpitations, episodes of dizziness, blood pressure dysregulation, tachycardia episodes and muscular pain.Her medical history indicated the patient was healthy overall but had had hip dysplasia and lumbar lordosis her entire life. At age 50, she contracted a campylobacter infection with long-term systemic inflammation, muscle and joint pain, upper body/head swelling and headaches. Magnetic resonance imaging showed L5-S1 disc degeneration and posterior bulge, an enlarged psoas bursa, bursitis of both hips, and osteoarthritis in both hips. Within a year after campylobacter infection, pain with eating, early satiation and epigastric pain became acute.
Methods and Procedures
Initial tests ordered by the internal medicine physician included: respiratory tests, nerve conduction studies of the lower extremities, blood analyses, and mesenteric duplex ultrasound. None of the tests suggested significant findings except the ultrasound, which showed elevated celiac artery velocities (334 cm/s at inspiration and 513 cm/s at expiration). Computed tomography angiography (CTA) with contrast dye confirmed severe compression of the proximal celiac artery.
The patient was referred to vascular medicine and gastrointestinal physicians and a surgeon for further evaluation, which included: a gastric emptying test; ultrasounds of the head and neck; computed tomography of the chest, abdomen with the pelvis, and neck; blood analysis; and endocrine tests. Physicians confirmed the MALS diagnosis, but they were not convinced that MALS was causing all the symptoms due to the lack of descriptive literature on MALS, so they continued ordering tests for 3 more months to rule out other conditions.
The total duration from onset of acute MALS-related symptoms following campylobacter infection to the diagnosis of MALS was 1 year, during which time the patient struggled to manage the symptoms that were causing significant distress and dysfunction.
She worked with a physical therapist (PT) to address symptoms associated with multiple system involvement in MALS and for sacroiliac dysfunction related to excessive lumbar lordosis, rigid psoas, L5-S1 disc degeneration, and hip dysplasia/arthritis. Evaluation found restriction and pain in the iliopsoas complex and diaphragm bilaterally. The lower rib cage was hypomobile with inhalation/exhalation and passive accessory movement testing.
The most beneficial PT strategies to reduce MALS-related symptoms included: instrument-assisted soft tissue mobilization; and strengthening and stretching to reduce lordosis (Fig. 1); manual lymphatic drainage; and primal reflex release techniques, such as the diaphragm lift, to mobilize the diaphragm[1]. The diaphragm lift resulted in immediate and long-term (2 weeks) reduction in dyspnoea and pain with eating.
As an occupational therapist, the patient integrated the PT home program into her activities of daily living by targeting habits and routines. For example, she did lymphoedema mobilization during hygiene (washing and drying) to move oedematous fluids toward lymphatic vessels and the heart.
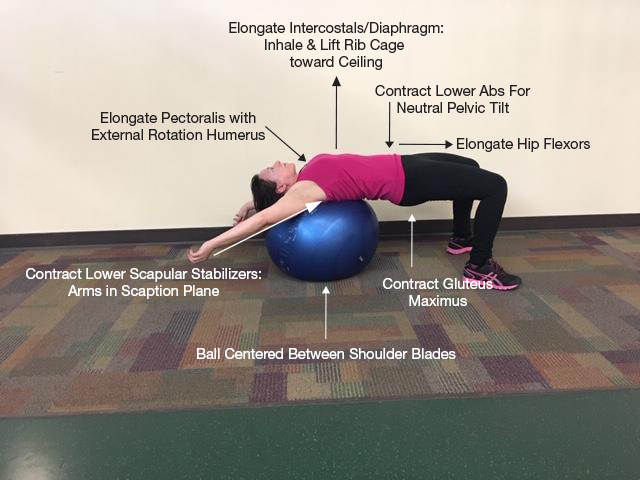
Table 1 (click to enlarge)
Figure 1. Strategies to reduce lumbar lordosis
During a time period when her diaphragm was most rigid, the patient experienced three episodes of rib subluxation. She went to a chiropractor who was able to reposition the rib(s), resulting in immediate pain reduction.
The patient then had laparoscopic surgery for median arcuate ligament (MAL) release with celiac plexus resection, which was carried out 15 months after the onset of acute symptoms and 38 years after the original onset of epigastric pain. The surgeon excised the ligament, resulting in visible improvement in the calibre of the celiac artery to a normal appearance. He noted significant inflammation in the MAL area.
Aside from the surgical pain, nausea and fatigue, the patient reported complete relief of all MALS-related pain and associated symptoms. She also lost 10 pounds of fluid within the first few days of surgery (Figs. 2 and 3).
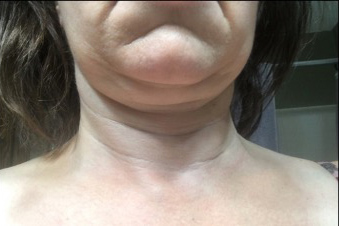
Figure 2 (click to enlarge)
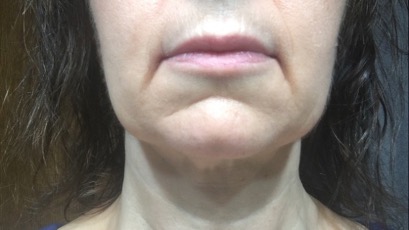
Figure 3 (click to enlarge)
Figure 2. Lymphoedema before surgery
Figure 3. Lymphoedema resolution after surgery
A year after MAL release surgery, the patient had increased hip arthritis, rigidity of hip flexors, and increased lumbar lordosis with a return of many of the original MALS-related symptoms but at lower levels of intensity. A repeated mesenteric ultrasound indicated celiac artery velocity to be elevated during expiration (228 cm/s) and normal during inspiration (102 cm/s), suggesting MALS. The patient then saw an orthopaedic surgeon and elected to have a total hip arthroplasty (THA).
Upon recovery from THA the patient was able to resume the stretching and toning exercises to reduce lumbar lordosis, which simultaneously resulted in complete resolution of all MALS-related symptoms. Table 1 provides complete descriptions of symptoms, management strategies and outcomes.
DISCUSSION
For this patient, there was a musculoskeletal connection between lumbar lordosis, hip dysplasia (and subsequent arthritis), and MALS as evidenced by parallel exacerbation and reduction of symptoms associated with each diagnosis. It is plausible that the congenital hip dysplasia irritated and shortened hip flexors, which led to lumbar lordosis with increased stretching and tightening of the MAL above the L1 vertebral attachment, putting pressure on the celiac nerves and artery. The inflammation from hip arthritis and secondary effects of campylobacter infection exacerbated the MALS-related symptoms. This offers a clue for more research into interprofessional management strategies for MALS, which includes strategies to reduce lumbar lordosis.
Other body systems are also affected by MALS as suggested by the wide array of symptoms in this patient, as follows:
- Nervous and cardiac: orthostatic hypotension and tachycardia from celiac nerve impingement/excitation [2]
- Gastrointestinal: decreased hydrochloric acid (HCl) and pancreatic enzymes [3]
- Lymphatic: lymphoedema and inflammation [4]
- Respiratory and vascular: dyspnoea; expiration causing increased compression of the celiac artery and blood flow velocities; organ pain with eating [5].
The fact that all symptoms were relieved after MAL release surgery affirms the assertion that MALS is a multisystem problem; hence, the MAL is the epicentre of dysfunction in multiple body systems that are affected by MALS. This warrants consultation with many healthcare providers to develop management strategies for MALS-related symptoms.
This is the only article that describes the extent of MALS symptoms and management strategies using the actual voice of the patient, which enhances authenticity. To manage bias, a panel of experts and direct healthcare providers of this patient contributed to case review and development of this article.
In conclusion, it is important to recognize the relationship between MALS, lumbar lordosis and hip dysplasia. In addition, MALS should be viewed as a multisystem problem that may respond to a variety of interprofessional management strategies provided to patients who are struggling to manage MALS-related symptoms for extended periods of time.
Patient Reflection
The vast array of MALS-related symptoms confused physicians who had never heard of MALS or who were looking exclusively for the classic triad (abdominal pain, weight loss and mid-epigastric bruit), causing delays with diagnosis and surgical intervention. The delays prompted the PT and me to use biomedical resources, clinical reasoning, and experimentation to develop conservative management strategies for MALS symptoms. More physician knowledge about MALS-related symptoms would have expedited the evaluation and intervention process by eliminating unnecessary tests (e.g. sleep study, hormones, cancer).
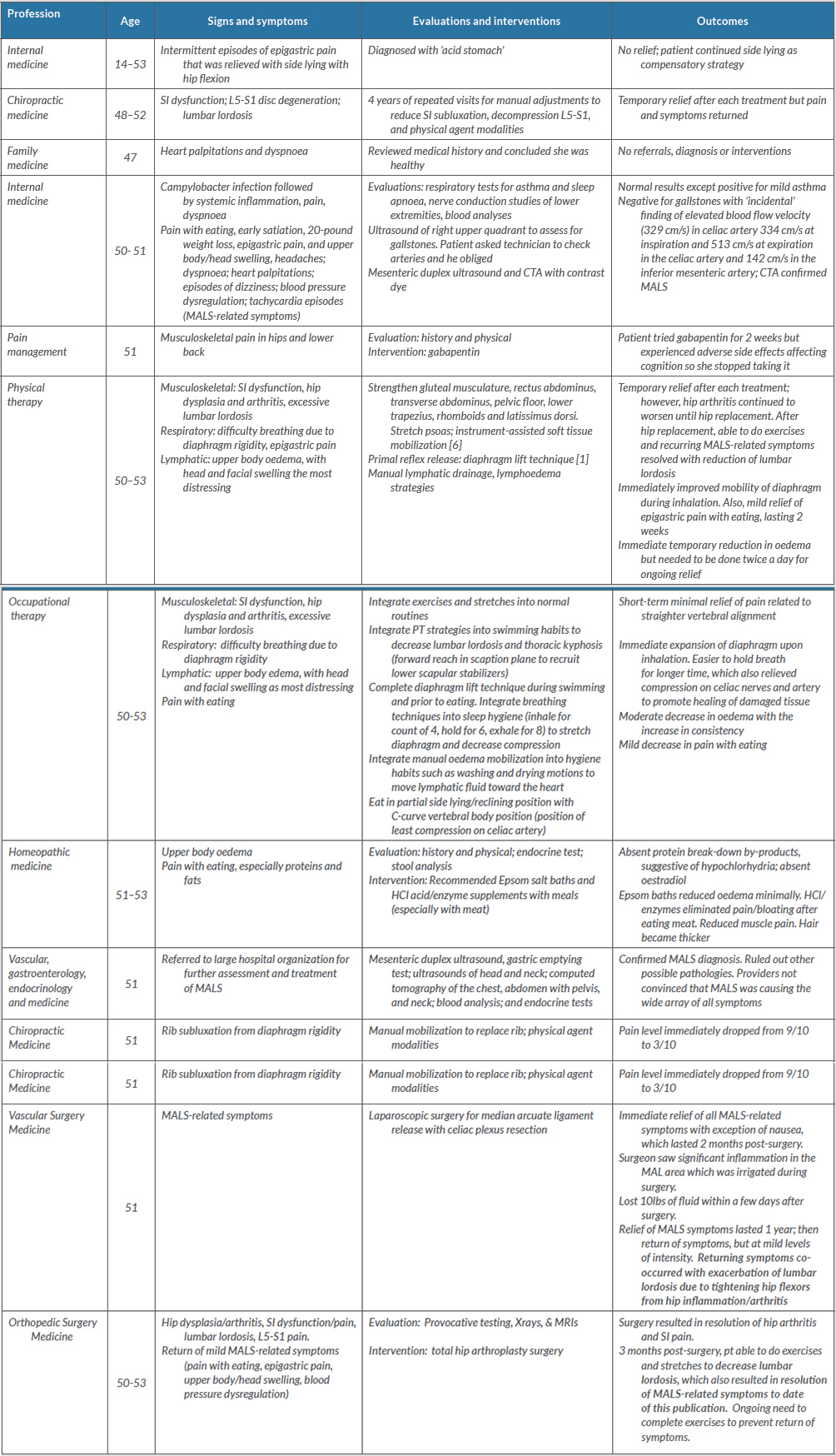
Table 1 (click to enlarge)
Table 1. Interprofessional management of median arcuate ligament syndrome (MALS)-related symptoms and lumbar lordosis and hip dysplasia
The table is organized by age of onset. ‘MALS-related symptoms’ include: upper abdominal pain with eating; early satiation; weight loss; epigastric pain; upper body/head swelling, especially in the left temporal area, causing headaches; dyspnoea; heart palpitations; episodes of dizziness; blood pressure dysregulation; tachycardia episodes; and muscular pain.
CTA, computed tomography angiography; MALS, median arcuate ligament syndrome; PT, physical therapy; SI, sacroiliac**
References
- Chapman EB, Hansen-Honeycutt J, Nasypany A, Baker RT, May J. A clinical guide to assessment and treatment of breathing pattern disorders in the physically active. Part I. Int J Sports Phys Ther 2016;11(5):803–809.
- Abdallah H, Thammineni K, Hartas G, Kane K. Median arcuate ligament syndrome presenting as POTS. Abstracts/Autonomic Neuroscience: Basic and Clinical 2015;192:56–141.
- Scholbach, T. Diagnosis and treatment of vascular compression syndromes of the abdomen based on the anatomical features of man and gender-specific characteristics after puberty, 28 May 2019. Available from: http://scholbach.de/wp-content/uploads/2017/09/20170917-vascular-compression-syndromes-website.pdf
- Dayan JH, Ly CL, Kataru RP, Mehrara BJ. Lymphedema: pathogenesis and novel therapies. Ann Rev Med 2018;69:263–276.
- Mak GZ, Speaker C, Anderson K, Stiles-Shields C, Lorenz J, Drossos T, et al. Median arcuate ligament syndrome in the pediatric population. J Pediatric Surg 2013;48(11);2261–2270.
- Rodriguez-Merchan EC, Corte-Rodriguez DL, Roman-Belmonte, JM. The current role of Astym therapy in the treatment of musculoskeletal disorders. Postgrad Med 2019;132(4):1–6.
APPENDIX
Literature Review - Interprofessional Management of Median Arcuate Ligament Syndrome Related to Lumbar Lordosis and Hip Dysplasia
Median arcuate ligament syndrome (MALS) is an under recognized condition in which the median arcuate ligament (MAL) compresses the celiac ganglion and celiac artery, causing pain, nausea, and multiple other vegetative symptoms. There are little to no non-surgical clinical intervention strategies described in the literature to support patients who struggle to manage the symptoms. Examination of the influence that lumbar lordosis has on the median arcuate ligament can support development of conservative management strategies for MALS. The purpose of this case study is to present interprofessional management strategies for further study in order to support patients with MALS.
Background
White et al. [1] described MALS, also called Dunbar syndrome or celiac artery compression syndrome, with symptoms that include: abdominal pain (not necessarily postprandial), weight loss, and bruit in the epigastric region that is more marked during expiration. There are differing theories related to the causative pathology of MALS, but ischemia and damage to organs from compression of the celiac artery may be prevented by a broad collateralization along the duodeno-pancreatico arterial arcade. The neurological sources of pathology in this syndrome is due to compression on the celiac ganglia. The cause of MALS is undetermined in the literature; it is most frequently described as a congenital anomaly of the aorta, celiac artery, and MAL [2] but there are no indications as to the cause of the anomaly. Scholbach [3] asserted that lordosis of the lumbar spine can lead to compression of various vessels in the abdomen, which is a potential cause of MALS.
Diagnosis of MALS usually takes an extensive time, with patients receiving little to no support for managing symptoms that disrupt functional performance in daily activities and quality of life due to severe pain. Symptoms are often nonspecific and diagnostic procedures are extensive, which delays diagnosis of MALS as physicians seek the underlying pathological condition [1]. Researchers in one study indicated variability of symptoms among 16 patients they studied, with only three patients presenting with the classic triad, which is postprandial abdominal pain, weight loss, and mid-epigastric bruit [4]. The authors added that three other patients had delayed diagnosis (range 12-60 months); they had been treated for anxiety first but the somatic symptoms persisted leading to the subsequent diagnosis of MALS [4]. When MALS is suspected, other conditions are excluded first before determining MALS is the cause of the symptoms [5]. Because of the variable symptoms presentations, involvement of multiple systems, and delay in diagnosis, patients struggle to manage the symptoms of MALS.
Diagnostic testing includes abdominal duplex ultrasound to measure velocities of blood moving through the celiac artery during inspiration and expiration and computed tomography angiography with inspiration and expiration to identify the changes in the celiac trunk morphology [6]. A celiac nerve block can determine nerve involvement if the celiac artery trunk is only slightly altered. Uncertainty remains if the celiac ganglion is mechanically altered too [5]. Researchers cautioned that pain caused by other entities affecting the celiac ganglion may respond equally.
The current treatment of MALS is surgery [1], which involves median arcuate ligament as well as partial celiac plexus resection. Surgeons are reporting favorable outcomes with laparoscopic procedures [7]. The only proposed conservative treatment is vasodilator medication for patients with mild symptoms [1], which lacks rationale and supportive evidence. There are currently no other identified interventions for alleviation of symptoms in the literature.
Multiple authors described MALS as being vascular problem and a nerve involvement problem [8, 9]. Limited studies [10,11] described and explained the wide variety of symptoms on the grounds of physiology and anatomy.
The authors of this article assert it is a multisystem problem, as evidenced by a wide variety of symptoms and comorbidities. Because of the involvement of multiple body systems, patients may experience a myriad of symptoms. The MAL is the epicenter of body dysfunction in people who have this condition, yet there are no evidence-based clinical intervention strategies to support patients in managing the myriad of symptoms associated with multiple body systems. In fact, MALS has been called a “vascular, nonvascular diagnosis” in response to the inconsistent alleviation of symptoms with either nerve plexus resection or revascularization [12] thus substantiating the need to consider less conventional approaches to treatment. To begin to generate such novel ideas for clinical intervention strategies, a review of body systems involvement with MALS is necessary.
Cardiac and Nervous system
Cardiac and nervous system effects are integrated in MALS cases. Researchers described MALS effects on the cardiac system by asserting the irritation of the celiac ganglion, humans’ largest sympathetic nervous plexus, causes a steady sympathetic drive responsible for cardiac symptoms such as tachycardia, dizziness, fainting, diarrhea or sweating [13]. Irritation to the celiac nerve plexus is exaggerated with minor physical or psychological efforts [13]. Thus, people with MALS will often report tachycardia and blood pressure drops during and after eating due to dumping of blood in dilated intestinal vessels. This can make patients with MALS feel exhausted, despite limited activity.
Researchers in another study also asserted that MALS causes impingement and excitation of the celiac plexus when they explored the relationship between MALS and postural orthostatic hypotension (OH) with tachycardia syndrome (POTS) among 41 patients who had both diagnoses [8]. Of these patients’ symptoms, half to all of them had typical MALS-related symptoms plus symptoms associated with the autonomic nervous system such as dizziness, clammy extremities, and headaches. Other researchers from a different study [14] assessed vagus nerve stimulation and the associated anti-inflammatory features (e.g. efferent cholinergic anti-inflammatory pathway) as a potential treatment for Crohn’s Disease. Consistent stimulation of the sympathetic celiac plexus inhibits parasympathetic nerve activity and may contribute to the inflammatory symptoms associated with MALS.
Vascular system
In another study, researchers examined collateral circulation of 37 patients with MALS in one healthcare organization and found that 32 of them were asymptomatic with 17 of them having arterial collateral circulations [15]. Of those with collateral circulations, nine had splanchnic artery aneurysms. In contrast, only one out of 20 patients without collateral circulations had an aneurysm.
Gastrointestinal system
Compression to the celiac artery can rarely cause ischemia to digestive organs, most often due to stenosis of the superior mesenteric artery. Nevertheless, compression of the MAL on the celiac plexus can also cause dysfunction, especially related to neurological signals for peristalsis and release of hydrochloric acid (HCL) and pancreatic enzymes [3].
Musculoskeletal system
Scholbach [3] is the only author who has described the musculoskeletal pathogenesis involved in MALS, which prevails in females. He indicated that in daily practice, over 90% of the afflicted ones are postpubescent girls or women. This is due to the prepubertal growth spurt and the specific changes of the female pelvis during puberty. Female puberty, in comparison to males, leads to a wider and deeper pelvis.
He asserted that women develop a significantly stronger lumbar lordosis because of a variety of specific changes[3]. Firstly, the greater depth of the pelvis causes its anterior tilt. Secondly, the wider hips stretch the basis of the psoas triangle, consisting of both psoas muscles and a virtual connecting line of their femoral insertion. Elongation of this basis with a constant length of both muscles forces a reduction of the height of this triangle. The height can be reduced only by a stronger anterior curvature of the lumbar spine [3].
Scholbach further explained both mechanisms add to the general human lordogenesis, which is unique in the evolution and is basically a consequence of the physiological overstretching of the hip joints [3]. Human hip joints, as all other mammalian hip joints, has a range of motion which is restricted by the tight ligaments of its capsule. A femoral position of 90° towards the lumbar spine is regarded the pendulum line of a resting hip joint. Unrestricted hip flexion and extension is then possible up to about 45°cranially and caudally to the pendulum line. So, a human newborn can never touch the mattress with its posterior knees in a supine position since the hips cannot yet be overstretched. Only with the beginning of human bipedal gait after about 12 months of age will the adult position of the hip joints gradually be achieved – and defined as normal. But this can be achieved only by overstretching the hip joints more caudally than 45°. Since a pure articular motion cannot yield such a position due to bondage of the femur by the ischiofemoral ligament, the only way to align the femur with the vortex-toe line is an anterior tilt of the human pelvis. This tilt also encompasses the sacral bone that is then producing the promontory, pointing then to the abdominal wall thus reducing the space for structures crossing the midline (left renal vein and left common iliac vein). The lumbar spine, attaching to the sacral bone has to compensate this anterior tilt by an equivalent dorsal curvature, resulting in the human lumbar lordosis. In summary, the unique human answer to gravitation is a lumbar lordosis, which causes several vascular compression syndromes (MALS, nutcracker syndrome, superior mesenteric artery syndrome, May-Thurner-syndrome, lumbar artery syndrome and others), predominantly in females [3].
Lymphatic system
There is no current literature describing the effects MALS has on the lymphatic system; therefore, older literature was included to help foster hypothetical reasoning. Many people with MALS experience malnutrition as a likely consequence of weight loss. The intermittent facial edema experienced by the patient could be related to protein deficiency [15]; the patient in this case report had a stool analysis that showed no protein breakdown byproducts despite having consumed protein. Other researchers explained that protein is needed for homeostasis as it maintains osmotic pressure to hold fluid inside blood vessels [16]. Reduced levels of protein, such as serum albumin, allow fluid to escape and collect within interstitial spaces, resulting in pitting oncotic edema. Edema can also be initiated and exacerbated by inflammation [17,18,19]. An extreme example of this condition is malnutrition-dependent fluid collection observed in the peritoneal cavity resulting in the bloated belly characteristic of Kwashiorkor [20]. A presentation of head and neck lymphedema is more commonly associated with surgical or radiation cancer interventions that damage the lymph vessels in this area [21]. None of these studies were related to MALS; therefore, the cause of edema in this patient is unknown because assumptions cannot be backed by facts that are related to MALS.
Respiratory system
The MAL attaches to the left and right crura, forming an arch above the celiac artery in most people; however, in people with MALS, the MAL is positioned lower on the aorta, often compressing the celiac nerves and artery, the first branch off the aorta below the diaphragm [1]. There is direct involvement of the respiratory system as evidenced by increased velocities of blood flow and increased compression of the celiac artery during expiration; reduced compression on the celiac artery and nerves is noted during inspiration [6].
Comorbidities
There was one robust retrospective analysis that compared comorbidities of 33,951 patients who had MALS with comorbidities of 1,029 patients who had undergone celiac artery decompression (CAD) surgery. Comorbidities for patients who had been diagnosed MALS were much higher and included conditions such as: hypertension (61.1%), peripheral vascular disorder (49.5%), fluid and electrolyte disorders (28.1%), chronic obstructive pulmonary disease (25.1%), and diabetes. Those who had CAD had much lower rates of comorbidities, with hypertension being the highest prevalence at 22.4% and the rest ranging from zero percent to 14%. Researchers of this study [23] asserted there is a need to educate physicians about these benefits of CAD surgery for patients with MALS. The high rates of comorbidities with MALS in this study [23] also showcases the extensive impact MALS has on multiple body systems.
Thus far, there are no rigorous quantitative studies or descriptive qualitative studies about interprofessional management strategies for MALS available in the literature. Most studies are focused on surgical intervention only and are conducted by single hospital organizations or providers, which raises bias related to positive outcomes rather than publishing of adverse complications and failures of surgery. An addition, there are no studies that examine the connection between MALS and musculoskeletal conditions, which may be potential causes of MALS. While there are many case studies presented in the literature from a provider/researcher perspective, none of them were authentic from the patient’s perspective, describing the vast array of symptoms and co-morbidities [23] that many patients experience and struggle to manage. The purpose of this case is to highlight interprofessional management of MALS related to lumbar lordosis and hip dysplasia in order to support patients who are waiting for MALS release surgery or who are not candidates for surgery.
Appendinx - References
- White RD, Weir-McCall JR, Sullivan CM, Mustafa SAR, Budak MF, Zealley IA. The celiac axis revisited: Anatomic variants, pathologic features, and implications for modern endovascular management. RadioGraphics 2015; 35, 879-98. doi: 10.1148/rg.2015140243.
- Skelly CL, Townsend E, Mak GZ. Median arcuate ligament syndrome. Rare Disease Database. National Organization for Rare Disorders 2019; Danbury, CT. Retrieved February 4, from: https://rarediseases.org/rare-diseases/median-arcuate-ligament-syndrome/
- Scholbach T. Diagnosis and treatment of vascular compression syndromes of the abdomen based on the anatomical features of man and gender-specific characteristics after puberty 2019; Retrieved May 28 from: http://scholbach.de/wp-content/uploads/2017/09/20170917-vascular-compression-syndromes-website.pdf
- Baccari P, Civilini E, Dordoni L, Melissano G, Nicoletti R, Chiesa R. Celiac artery compression syndrome managed by laparoscopy. Society for Vascular Surgery 2009; 50(1); 134-139. doi:10.1016/j.jvs.2008.11.124.
- Sun Z, Fritz DA, Turner S, Hardy DM, Meiler SE, Martin DC, Dua A. Celiac plexus block as a predictor of surgical outcome for sympathetically mediated abdominal pain in a case of suspected median arcuate ligament syndrome: a case report. Anesthesia & Analgesia Practice 2018; 11(3), 76-78. doi: 10.1213/XAA.0000000000000743.
- Mak GZ, Speaker C, Anderson K, Stiles-Shields C, Lorenz J, Drossos T, Liu D, Skelly CL. Median arcuate ligament syndrome in the pediatric population. J of Pediatric Surg 2013; 48(11), 2261-70. doi: 10.1016/j.jpedsurg.2013.03.003.
- El-Hayek M., Titus J, Bui A, Mastracci T, Kroh M. Laparoscopic median arcuate ligament release: are we improving symptoms? J Am Coll Surg 2013; 216, 272-9.
- Abdallah H, Thammineni K, Hartas G, Kane K. Median arcuate ligament syndrome presenting as POTS. Abstracts/Autonomic Neuroscience: Basic and Clinical 2015; 192: 56-141. doi: 10.1016/j.autneu.2015.07.220.
- Sultan S, Hynes N, Elsafty N, Tawfick W. Eight years experience in the management of median arcuate ligament syndrome by decompression, celiac ganglion sympathectomy, and selective revascularization. Vasc Endovascular Surg 2013; 47: 614-9.
- Klimas A, Lemmer A, Bergert H, Brodhun M, Scholbach T, Großer K. Laparoscopic treatment of celiac artery compression syndrome in children and adolescents. Vasa 2015; 44(4):305-12. doi: 10.1024/0301-1526/a000446. PubMed PMID: 26314363.
- Scholbach T. Celiac artery compression syndrome in children, adolescents, and young adults: clinical and color duplex sonographic features in a series of 59 cases. Journal of Ultrasound Medicine 2006; 25(3):299-305. PubMed PMID: 16495489.
- Skeik N, Cooper LT, Duncan AA, Jabr FI. Median arcuate ligament syndrome: a nonvascular, vascular diagnosis. Vasc Endovascular Surg. 2011, July;45(5):433-7. doi:10.1177/1538574411406453. Epub 2011 May 2.
- Klimas A, Lemmer A, Bergert H, Brodhun M, Scholbach T, Groβer K. Laparoscopic treatment of celiac artery compression syndrome in children and adolescents. VASA. 2015; 44: 305-312. doi: 10.1024/0301-1526/aooo446.
- Bonaz B, Sinniger V, Pellissier S. Vagus nerve stimulation: A new promising therapeutic tool in inflammatory bowel disease. Journal of Internal Medicine 2017; 282(1): 46-63. doi: 10.1111/joim.12611.
- Heo S, Jin Kim H, Kim B, Keun J. Clinical impact of collateral circulation in patients with median arcuate ligament syndrome. Diagnostic and Interventional Radiology 2018; 24(4): 181-186. doi: 10.5152/dir.2018.17514.
- Samonds KW, Hegsted DM. Protein deficiency and energy restriction in young cebus monkeys. Proc Natl Acad Sci U S A. 1978; Mar;75(3):1600-4.
- Mortimer P S, Rockson S. New developments in clinical aspects of lymphatic disease. J of Clin Invest 2014; 124(3), 915-921. doi: 10.1172/JC171608.
- Dayan JH, Ly CL, Kataru RP, Mehrara BJ. Lymphedema: Pathogenesis and novel therapies. Annu Rev Med. 2018; Jan 29;69:263-276. doi: 10.1146/annurev-med-060116-022900.
- Ly CL, Kataru RP, Mehrara BJ. Inflammatory manifestations of lymphedema. Int J Mol Sci. 2017; Jan 17;18(1). pii: E171. doi: 10.3390/ijms18010171.
- Rockson SG, Tian W, Jiang X, Kuznetsova T, Haddad F, Zampell J, Mehrara B, Sampson JP, Roche L, Kim J, Nicolls MR. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight 2018; Oct 18;3(20). pii: 123775. doi: 10.1172/jci.insight.123775.
- Coulthard MG. Oedema. Kwashiorkor is caused by hypoalbuminaemia. Paediatr Int Child Health 2015; May;35(2):83-9. doi: 10.1179/2046905514Y.0000000154. Epub 2014 Sep 16.
- Yuan Y, Arcucci V, Levy SM, Achen MG. Modulation of immunity by lymphatic dysfunction in lymphedema. Front Immunol 2019; Jan 29;10:76. doi: 10.3389/fimmu.2019.00076. eCollection 2019.
- Rezigh AB, Desai SS, Afifi RO, Charlton-Ouw KM, Miller CC, Estrera AL, Safi HJ, Azizzadeh A. Celiac artery decompression for median arcuate ligament syndrome: a US national inpatient sample study. Abstract for the annual symposium for the society for clinical vascular surgery 2015; March 29-April 2. Miami. Florida. US. Retrieved May 29, 2019 from: http://scvs.org/symposium/abstracts/2015/32.cgi



