ABSTRACT
Case presentation: A 50-year-old female presented with an onset of multiple subcutaneous nodules on her 4 limbs. These nodules appeared concomitantly with the initiation of radioactive iodine therapy for papillary thyroid cancer. These nodules were not obvious on inspection of the skin, but easily felt on palpation.
The biopsy of the subcutaneous nodules revealed hypodermic non-caseating granulomas consistent with sarcoidosis. The patient underwent an 18F-fluorodeoxyglucose positron emission tomography (PET) scan study that revealed, besides the subcutaneous nodules, multiple hypermetabolic mediastinal lymphadenopathies and cervical adenopathies. Biopsy of the mediastinal lymphadenopathy showed neither granulomas nor neoplastic cells. Cervical biopsy revealed neoplastic cells of thyroid origin. Laboratory tests were normal. Bronchoalveolar lavage showed a normal CD4/CD8 T-cell ratio.
A diagnosis of cutaneous sarcoidosis was established, as well as a recurrence of the cancerous disease. The subcutaneous nodules regressed spontaneously in the absence of any treatment.
Discussion and conclusion: Sarcoidosis is a multisystemic disease of unknown origin. This case illustrates an uncommon occurrence of sarcoidosis, triggered by radioactive iodine therapy. Radioiodine may lead to immunological changes, especially affecting the Th1/Th2 ratio, which may promote the emergence of sarcoidosis in genetically predisposed patients. There is still much to discover to fully understand the pathogenesis of sarcoidosis.
LEARNING POINTS
- The immunopathogenesis of sarcoidosis is poorly understood, as well as the environmental factors involved.
- Radioactive iodine therapy for thyroid cancer treatment may be an environmental trigger.
- The immunological changes induced by radioiodine, especially with respect to the Th1/Th2 ratio, may promote occurrence of sarcoidosis in genetically predisposed patients.
KEYWORDS
Sarcoidosis, radioiodine therapy, thyroid cancer
CASE DESCRIPTION
A 50-year-old woman was admitted to internal medicine consultation for the evaluation of subcutaneous nodules. At the end of November 2018, the patient underwent a total thyroidectomy with lymph node dissection for a follicular variant papillary carcinoma (pT2N1bM0). The positron emission tomography (PET) for disease extension was negative. Treatment with radioactive iodine-131 (I-131) was initiated in January 2019.
The day after initiation of I-131 therapy, the patient reported the progressive appearance of multiple subcutaneous nodules bilaterally on the lower and upper limbs as well as at the abdominal level, accompanied by an episode of shivering and diffuse arthralgia mostly on the feet. The patient was treated with non-steroidal anti-inflammatory drugs, resulting in a modest improvement. She was seen in April 2019 at the internal medicine consultation for further assessment.
The patient was known to have high blood pressure, hypercholesterolaemia and type 2 non-insulin-dependent diabetes mellitus. She underwent surgery for a secreting adrenal adenoma as well as for a liposarcoma of the thigh in 2012. She worked as a secretary, did not own any pets and had not travelled outside Belgium during the previous 5 years.
Her usual treatment included Glucophage, amlodipine, perindopril, allopurinol, rosuvastatin and the intake of levothyroxine and hydrocortisone.
The evolution since the beginning of the symptomatology was rather favourable with a progressive decrease in joint pain. Skin lesions were still present but not painful.
During physical examination, a straight and indurated lateral cervical lymphadenopathy of approximately 2 cm was revealed, as well as numerous subcutaneous nodules of approximately 1 to 2 cm, painless and with normal skin in sight. The lesions were located in the 4 limbs as well as at the abdominal level. The rest of the physical examination was normal.
The blood test showed no inflammatory syndrome. At the level of the haemogram, we observed an isolated lymphopenia at 1,100 lymphocytes/mm³ (normal 1,300–4,500/mm³). Liver tests, kidney function and the ionogram were normal. There was a hypercalcaemia at 2.67 mmol/l (normal 2.20–2.60 mmol/l) with normal albumin levels at 40 g/l (normal 34–46 g/l). Parathyroid hormone and vitamin D were normal. Protein electrophoresis was normal.
Testing for rheumatoid factor, antinuclear factor and antineutrophil cytoplasmic antibodies was negative. The levels of angiotensin-converting enzyme were normal.
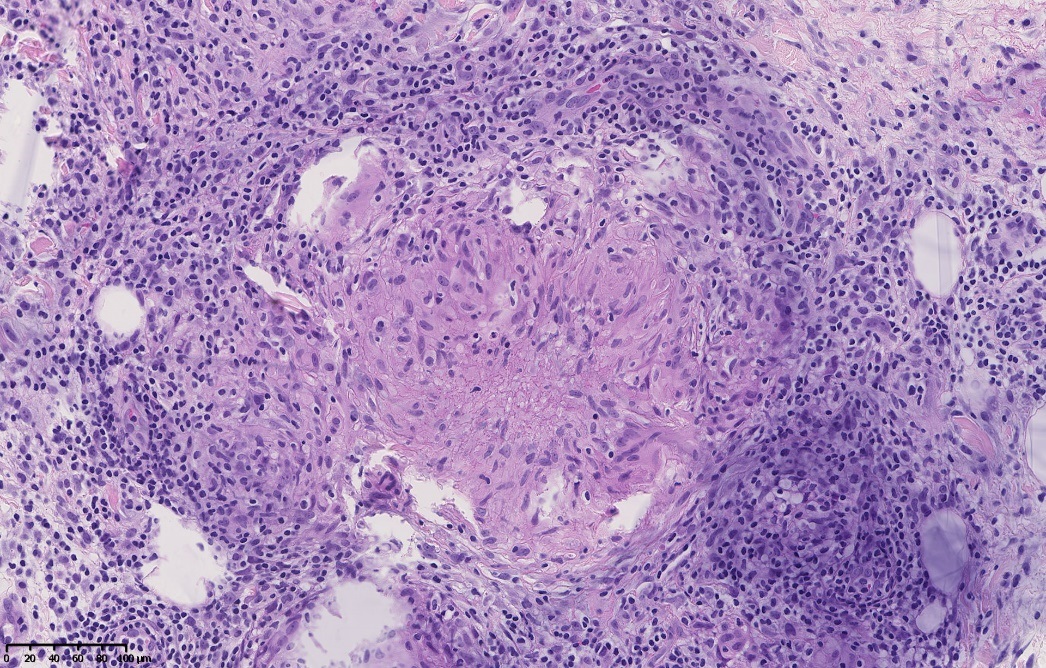
Skin biopsy revealed the presence of a mainly mononuclear inflammatory infiltrate at the hypodermic level, consisting of many histiocytes forming granulomatous epithelioid nodules, which were occasionally necrotizing (Fig. 1). These granulomas were surrounded by a dense lymphocytic crown. Wade-Fite, Ziehl-Neelsen, Grocott and periodic acid-Shiff staining did not reveal the presence of microorganisms. Vascular tropism has been ruled out by Miller stain and immunohistochemical technique (ETS-related gene). Culture carried out to investigate the presence of mycobacteria was negative.
Figure 1. The biopsy of the subcutaneous nodules showing hypodermic non-caseating granulomas
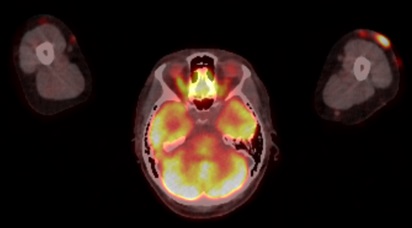
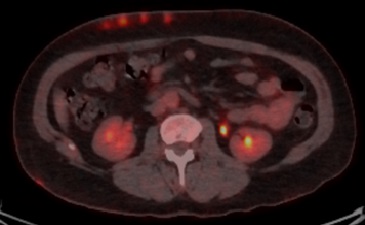
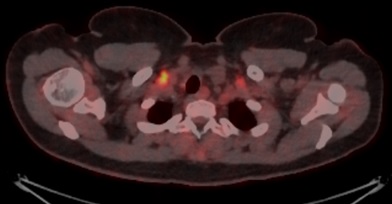
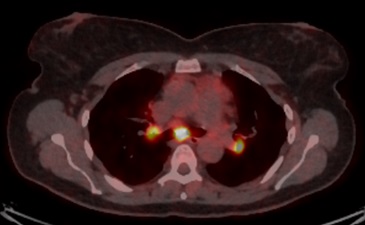
The patient underwent a PET scan, which showed hypermetabolic subcutaneous lesions (Figs. 2 and 3), as well as right latero-cervical hypermetabolic lymphadenopathy (Fig. 4) and multifocal mediastinal adenopathies (Fig. 5). A chest scan was performed, confirming the presence of mediastinal lymphadenopathies, without parenchymal involvement. Functional respiratory tests were within normal limits.
Figure 2. PET scan showing hypermetabolic subcutaneous nodules on the upper limbs
Figure 3. PET scan showing hypermetabolic subcutaneous nodules at the abdominal level
Figure 4. PET scan showing cervical adenopathies
Figure 5. PET scan showing hypermetabolic mediastinal lymphadenopathies
Endobronchial ultrasound (EBUS) was not very helpful but did not show any neoplastic cells or granulomas. Bronchoalveolar lavage showed a normal CD4/CD8 T-cell ratio at 1.42 (normal 1–4).
Based on the patient’s history, a cervical lymph node puncture was performed, showing neoplastic cells of thyroid origin.
A diagnosis of cutaneous sarcoidosis was established, as well as a recurrence of her cancerous disease at the cervical level.
In the absence of any pulmonary involvement, and considering the low clinical impact, no treatment was issued for the sarcoidosis. The subcutaneous nodules gradually disappeared spontaneously over the following months and calcium levels normalized. The patient was referred to oncology for further treatment.
DISCUSSION
Our case report illustrates the occurrence of a predominantly cutaneous systemic granulomatosis following treatment with I-131 for thyroid carcinoma. The diagnosis of cutaneous sarcoidosis is retained taking into account the presence of typical rare cutaneous lesions which can be considered part of Darier-Roussy syndrome, whilst excluding any other granulomatous disease. Stage I mediastinal involvement is strongly suspected, but unfortunately, EBUS has not confirmed this. Darier-Roussy syndrome is a form of cutaneous sarcoidosis characterized by an infiltration of nodules in the subcutaneous fat, and is of fairly rare prevalence: 1 to 6% [1, 2].
The originality of the case is the temporal relationship of the onset of the disease with I-131 treatment, suggesting a causal link.
The immunopathogenesis of sarcoidosis is complex and not yet fully understood. It is recognized that it is mainly a Th1 cell-mediated immune response, although recently the role of Th17 has been established, particularly in pulmonary involvement [3]. The activation and proliferation of CD4 T cells, Th1-polarized, leads to the formation of granulomas via the activation of macrophages with the involvement of various cytokines: tumour necrosis factor (TNF)-α, interleukin (IL)-2, IL-12, IL-17 and interferon (IFN)-γ [4, 5].
I-131 therapy could be an environmental trigger causing immunological disturbances, precipitating the formation of sarcoid granulomas in genetically predisposed individuals. In 2015, Simonovic et al. conducted a study to measure the levels of Th1 and Th2 cytokines before and 7 days after treatment with I-131 in patients with differentiated thyroid cancer [6]. A significant decrease in the production of Th2 cytokines was shown after treatment. Although the Th1/Th2 cytokine ratio was not significantly different before and after treatment, this study showed that I-131 therapy induces an immunological change with a tendency towards decreased Th2 cytokine levels.
In 2010, Xiang et al. showed that treatment with iodine-125 (I-125), another iodine radioisotope, for hepatocarcinoma, changes the Th1/Th2 balance [7]. Th1 cytokines, such as IFN-1 and IL-12, are present at a significantly higher concentration after treatment with I-125, compared to the control group and to pre-treatment levels.
These two studies show that radioiodine therapy leads to immunological changes with a tendency to modify the Th1/Th2 balance in favour of an excess of Th1 cytokines. These immunological changes could precipitate the formation of sarcoid granulomas in genetically predisposed patients.
To the best of our knowledge, there is only 1 other case in the literature reporting the occurrence of sarcoid lesions after treatment with I-131. Myint and Chow described a case of the recurrence of sarcoidosis occurring after I-131 therapy for thyroid carcinoma [8]. The sarcoidosis disease of the patient was considered to be in remission for many years, until she developed a bone lesion after I-131 therapy for thyroid cancer. The bone biopsy revealed non-caseating granulomas consistent with the recurrence of sarcoidosis disease.
CONCLUSION
This is the second case in the medical literature to report the occurrence of cutaneous sarcoidosis after treatment with radioiodine for thyroid cancer. Radioiodine therapy may lead to immunological changes, especially in the Th1/Th2 ratio, which may promote the emergence of sarcoidosis in genetically predisposed patients. Additional studies are required for further elucidation.