ABSTRACT
Acquired haemophilia (AHA) is a rare autoimmune disorder caused by circulating autoantibodies that inhibit the activity of factor VIII (FVIII). Acquired inhibitors against FVIII are rarely seen, with a reported incidence of approximately 1 case per million/year. Clinical conditions and contexts associated with AHA include autoimmune diseases, lymphoproliferative malignancies, drug treatment, pregnancy and infections. An association with urticarial vasculitis is even more rare. Here, we report a case of a 59-year-old woman presenting with cutaneous and muscle haematomas secondary to AHA in association with urticarial vasculitis, who was successfully treated with factor eight inhibitor bypassing activity (FEIBA) and prednisolone.
LEARNING POINTS
- Acquired haemophilia (AHA) is a rare autoimmune disease.
- AHA predisposes to severe, potentially life-threatening haemorrhage.
- An association with urticarial vasculitis is even more rare.
KEYWORDS
Haemophilia, urticarial vasculitis
INTRODUCTION
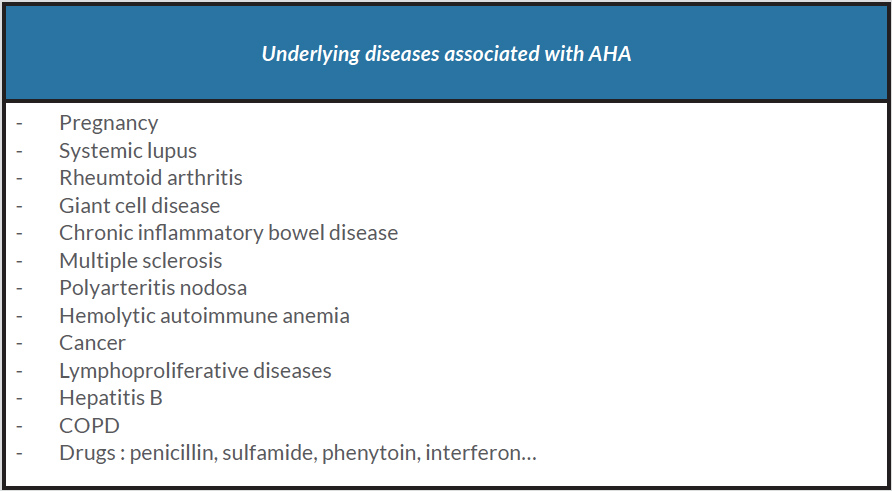
Acquired haemophilia (AHA) is a rare autoimmune disease caused by autoantibodies against factor VIII (FVIII). The average incidence is 1 case/million/year and increases with age. Although rare, it is still the most common acquired factor deficiency. Typically, it manifests as unexpected, abrupt and unusually severe bleeding events in a patient with no known personal or familial history of bleeding. The severity of bleeding is variable and can include subcutaneous, soft tissue, retroperitoneal, gastrointestinal and intracranial haemorrhage. The diagnosis is confirmed by laboratory investigations with a prolonged activated partial thromboplastin time (APTT), low FVIII levels and high FVIII inhibitor levels. The aetiological assessment is disappointing in almost two-thirds of cases [1]. In other cases, there is a context of autoimmune disease, pregnancy, malignancies, infections or drug treatment (Table 1). Treatment which consists of haemostatic management and eradication of the inhibitors can be challenging to manage. Outcome can be fatal, mainly during the first few weeks following diagnosis.CASE DESCRIPTION
The patient was a 59-year-old Caucasian woman with a medical history of chronic hypertension treated with amlodipine. She presented to the emergency department complaining of multiple ecchymoses and abdominal pain, which had been progressing for several days. She denied any history of trauma, abnormal bleeding, a familial history of bleeding disorders or use of any anticoagulant medications.
The previous month, she had presented a diffuse maculopapular rash. The skin biopsy showed leucocytoclastic vasculitis and the patient was diagnosed with urticarial vasculitis. There was no other impairment such as renal or pulmonary involvement. The aetiological assessment was negative. The patient was treated with prednisolone for 3 weeks with a rapid, good outcome.
On clinical examination, the patient was in good general condition, BP 123/60, pulse 85 and temperature 37°C. There was a large subcutaneous haematoma occupying the whole of the left upper limb, a superficial haematoma on the right calf and numerous ecchymoses around the 2 ankles. She denied bleeding from the nose or gastrointestinal tract. The patient also reported pain in the left iliac area without psoitis. She had no adenopathy and her spleen was nonpalpable.
A complete blood count (CBC) revealed a haemoglobin (Hb) level of 8.8 g/dl, and a platelet count of 540 G. A coagulation profile showed a prolonged APTT of 107 seconds, a normal prothrombin time (PT) and a normal international normalized ratio. The results of the FVIII assay were low at 1% (N: 70–100) and inhibitor levels were elevated at 16 Bethesda units (BU), suggestive of AHA. As the patient complained of abdominal pain, an abdominal CT scan was performed and showed a large haematoma on the left psoas measuring 47×12 mm.
Screening for malignancies, lymphoproliferative disease and autoimmune disorders was negative.
Treatment was started with factor eight inhibitor bypassing activity (FEIBA) 100 U/kg twice daily and prednisolone 2 mg/kg/day with a good outcome. The different haematomas reduced in size, and the patient did not experience any further bleeding during the hospitalization. Ten days later, the FVIII level increased to 55% and the anti-VIII inhibitor level decreased to 2 BU. After 6 weeks, the coagulation profile revealed an APTT of 28 seconds and a FVIII level of 61%. There was no need for either cyclophosphamide or rituximab.
The patient was later discharged on a prednisone taper and with an outpatient haematology follow-up.
DISCUSSION
AHA is considered to be an anticoagulation defect secondary to the presence of inhibitory autoantibodies against FVIII. The first case of AHA was described in 1940 by Lozner et al., in an elderly male who suffered from major bleeding after a surgery [1].
Prognosis depends on the clinical course, the severity of bleeding, the underlying cause and the presence of comorbidities. Spontaneous resolution occurs in 25% of patients, mainly in those with medication- or pregnancy-induced AHA [2].
Management of AHA involves 2 fundamental steps: controlling the acute haemorrhage and long-term eradication of autoantibodies by using immunosuppressive agents. Two strategies are available for the treatment of acute haemorrhage: the use of bypassing agents or raising the level of circulating FVIII.
Various immunosuppressive agents and combinations of these are used for the elimination of FVIII autoantibodies. Glucocorticoids alone or glucocorticoids with either cyclophosphamide or rituximab are primarily used in this regard. Other treatment options, including high-dose intravenous immunoglobulins and plasmapheresis, have been used along with immunosuppressive agents for refractory patients.
AHA is considered idiopathic in more than half of cases. A study based on a European registry of 501 patients with AHA [3] and a UK study of 172 patients [4] found no underlying disease in 52% and 65% of patients, respectively. For patients with an identified cause, the most common causes were malignancy (12% and 15%) and autoimmune disease (12% and 17%).
The association between AHA and urticarial vasculitis is therefore very rare. Cutaneous vasculitis tests were negative, including the drug investigation. The haemorrhagic syndrome occurred a few days after completing steroid therapy. The patient presented with both skin haematomas and muscle involvement with the left psoas. The evolution was very rapidly favourable under treatment combining activated prothrombin complex concentrate (FEIBA) and prednisolone.
To the best of our knowledge, this is the third reported case of autoimmune haemophilia associated with urticarial vasculitis. Usually, an association between AHA and systemic vasculitis is very rare, with only a few case reports in the literature; these featured giant cell arteritis [5], ANCA-associated vasculitis [6] and polyarteritis nodosa [7, 8]. Cases associated with urticarial vasculitis are even more rare; only 2 cases have been published up to now. A case was reported by Christiansen et al. [9] with a 71-year-old patient who developed urticarial vasculitis 5 months after the diagnosis of AHA. The cutaneous vasculitis was treated with azathioprine for 3 years. In 2006, Patel et al. [10] reported on a 66-year-old patient who had been treated for 4 years for urticarial vasculitis, who was still on 10 mg prednisolone, and who developed AHA, with a favourable outcome after an increase in steroid therapy.
In conclusion, AHA, although easily diagnosed, remains a mysterious disease with various associations, including some cases of vasculitis.