ABSTRACT
Aortoenteric fistula (AEF) is a rare condition with a high mortality rate. AEFs are classified according to their primary and secondary causes, the former being less frequent. Primary AEFs occur in a native aorta and their causes include aneurysms, foreign bodies, tumours, radiotherapy and infection. The classic triad of aortoesophageal fistulas, a subtype of AEFs, are mid-thoracic pain and sentinel haemorrhage, followed by massive bleeding after a symptom-free interval.
We present the case of a 41-year-old male patient who presented in the emergency room after successive episodes of abundant haematemesis. He was hypovolemic, hypothermic and acidotic at presentation. His medical history included an emergency room visit the week before with chest pain but no relevant anomalies on work-up, active intravenous drug use and chronic hepatitis.
Esophagogastroduodenoscopy (EGD) showed a bulging ulcerated lesion suspicious for aortoesophageal fistula, confirmed by computed tomography (CT) angiography, which revealed a saccular aortic aneurysm with a bleeding aortoesophageal fistula. The patient underwent urgent thoracic endovascular aortic repair.
The sentinel chest pain, leucocytosis and CT findings hinted at the presence of a mycotic aneurysm, despite the negative blood cultures. It was most likely caused by a septic embolus due to the patient’s risk factors. While a high level of suspicion for aortoesophageal fistula is needed to prompt a fast diagnosis, EGD and CT findings were crucial to establish it and allow a life-saving intervention. We conclude that chest pain cannot be disregarded in a patient aged 41 years with multiple comorbidities, despite normal work-up, to prevent a fatal outcome.
LEARNING POINTS
- Aortoesophageal fistula is a rare cause of severe upper gastrointestinal haemorrhage with a high mortality rate.
- Computed tomography angiography is diagnostic in most cases but a high level of suspicion is essential.
- Chest pain, a characteristic clinical symptom of aortoesophageal fistula, cannot be disregarded in a patient with multiple comorbidities, even in the presence of a normal electrocardiogram and chest x-ray.
KEYWORDS
Aortoesophageal fistula, mycotic aneurysm, computed tomography angiography
CASE DESCRIPTION
A 41-year-old patient presented in the emergency room upon waking up feeling nauseated and bloated. He presented successive episodes of abundant haematemesis of bright red blood followed by syncope. One week before admission, he had attended the emergency room with a main complain of chest pain irradiating to the left shoulder. Work-up showed elevated leucocytes (20,200×106/l) and C-reactive protein (CRP; 48 mg/l), haemoglobin of 13.0 g/l, and a urinary tract infection for which empiric antibiotherapy (fosfomycin) was prescribed. An electrocardiogram and chest x-ray were normal.
The patient’s relevant medical history included active intravenous heroin use, smoking and alcoholic habits, knee septic arthritis complicated by a lumbar abscess (6 years previously), and chronic hepatitis C. He had a temperature of 34.5ºC, blood pressure of 60/30 mmHg and heart rate of 110 bpm.
METHODS AND PROCEDURES
Blood tests showed decreased haemoglobin (9.5 g/l) and haematocrit (0.28 L/L), and elevated mean cell haemoglobin (33.9 pg) and leucocytes (21,100×10 /l; 79% neutrophils). CRP was 11 mg/l. The patient was acidotic, with a pH of 7.225, pCO2 43.8 mmHg, and HCO3 18.1 mmol/l. Lactates were elevated (9.6 mmol/l). Toxicology assays were positive for marijuana and benzodiazepine.
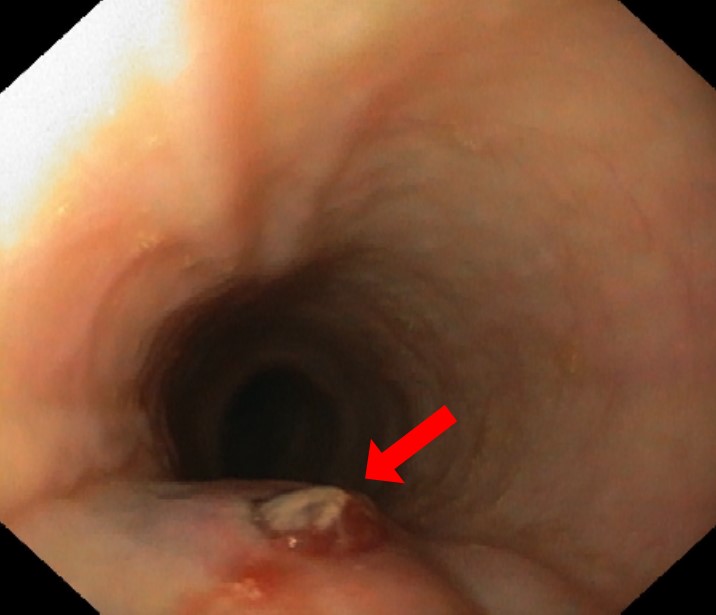
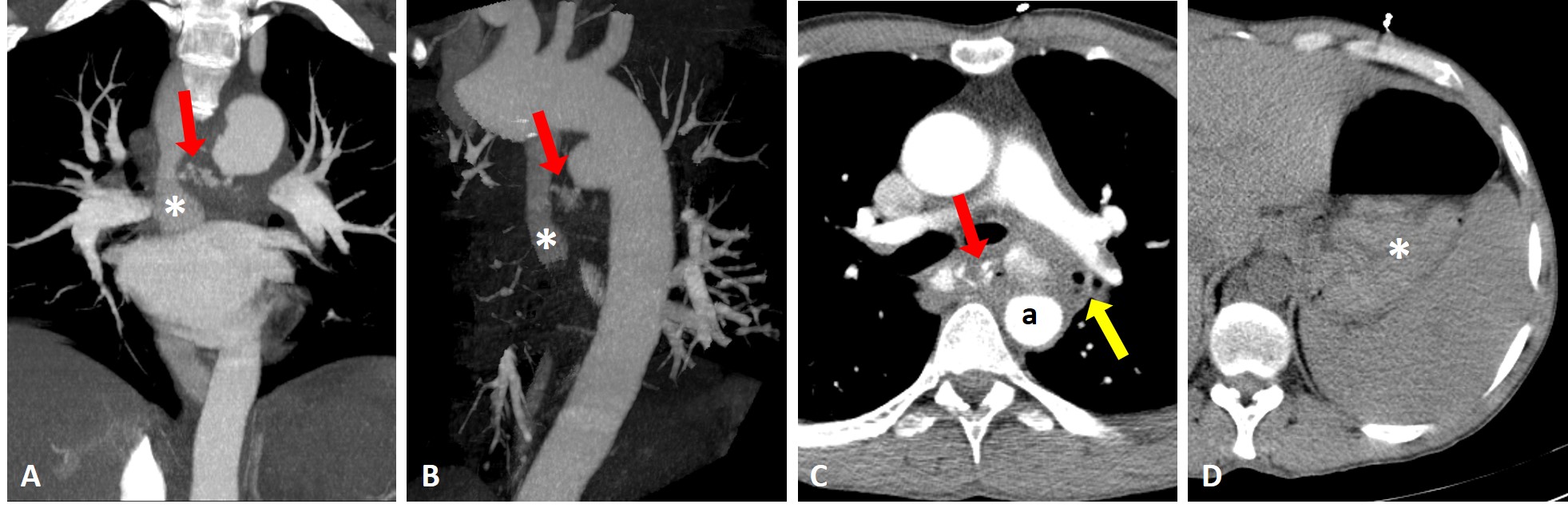
An esophagogastroduodenoscopy (EGD) was performed, which was inconclusive due to blood along the oesophagus and stomach. The patient was admitted to the intensive care unit, and intravenous octreotide, proton pump inhibitors and erythromycin were initiated. The EGD was repeated 6 hours later revealing a bulging ulcerated lesion with an adherent clot 28 cm below the incisors (Fig. 1). These findings raised the suspicion of an aortoesophageal fistula, which was confirmed with computed tomography angiography, revealing a saccular aortic aneurysm with a bleeding tortuous aortoesophageal fistula (Fig. 2).
The patient was referred to vascular surgery and underwent urgent thoracic endovascular aortic repair with the deployment of a Cook-stent graft with a favourable outcome. Intravenous empiric treatment with amoxicillin–clavulanate, ampicillin and metronidazole was initiated despite negative blood cultures.
Figure 1. Esophagogastroduodenoscopy performed in the setting of massive upper gastrointestinal bleeding, revealed a bulging ulcerated lesion, with an adherent clot 28 cm below the incisors (arrow)
Figure 2. Coronal (A) and oblique sagittal (B) maximum intensity projection reconstructions of aortic computed tomography (CT) angiography, revealing a communication between a saccular aortic aneurism and the oesophagus (red arrow). The oesophageal lumen filled with vascular contrast (asterisk). (C) Enhanced axial thoracic CT showing effacement of the periaortic fat and ectopic gas adjacent (yellow arrow) to the aortic lumen (a). (D) Abdominal axial CT before contrast with spontaneously dense content inside the stomach (asterisk)
Three days after surgery, the patient presented low haemoglobin and melaena. EGD showed a 1 cm wide laceration of the oesophagus with friable borders, which was successfully closed with through-the-scope clips.
Thirteen days after surgery, the patient was febrile and had elevated CRP (49 mg/l). Blood and central venous catheter cultures were positive for a multidrug-resistant Klebsiella pneumoniae, successfully treated with direct antibiotherapy for 24 days. A CT scan showed a normally positioned stent with no signs of infection. The patient was discharged with 75 mg of clopidogrel daily.
DISCUSSION
AEF is a rare but potentially fatal entity, with an associated mortality of 77% with treatment and 100% fatal without intervention [1]. Hence, an early diagnosis is crucial to increase the chances of survival.
AEFs are divided into those with primary and with secondary causes. Most cases are secondary to vascular interventions, in the setting of prior aortic surgery or graft placement. Primary AEFs occur in a native aorta and are considerably less frequent than secondary [2]. Causes include aneurysms (most common), foreign bodies, tumours, radiotherapy and infection (classically tuberculosis and syphilis) [2]. The estimated annual incidence of primary and secondary AEFs is about 0.0015% and 0.6–2%, respectively [1].
The oesophagus is involved in about 28–30% of AEFs [2]. The classic triad of symptoms of aortoesophageal fistulas are mid-thoracic pain and a sentinel haemorrhage, followed by massive bleeding after a symptom-free interval. Such herald bleeding (self-limited) is reported in up to 50–90% of patients [1, 2]. This patient had attended the emergency room 1 week previously with a main complain of chest pain, which we retrospectively presumed to be sentinel haemorrhage, supported by the 3.5 g drop in haemoglobin between the two emergency room visits. But, at attendance, there were no other signs that could have prompted this suspicion, since there was no history or signs of haematemesis, and a normal haemoglobin, electrocardiogram and chest x-ray.
EGD is the initial diagnostic tool for upper gastrointestinal bleeding, but its sensitivity for AEF is low, as a pulsatile bulging lesion with or without adherent blood clots is rarely found [1, 2]. The findings on the second EGD were highly suspicious for AEF, and CT angiography was necessary for confirmation. It revealed the classic AEF signs, ectopic gas adjacent to the aortic lumen and effacement of the periaortic fat plane, but also more characteristic and rare signs of direct extravasation of vascular contrast into the oesophagus [3].
In this patient, the chest pain, increased CT attenuation of the periaortic fat, ectopic gas and aortic bulging, together with leucocytosis, hinted at the presence of a mycotic aneurysm. A lack of fever and negative blood culture are not enough to rule out an infected aneurysm, as up to 50% of cultures are negative [4].
Aortic infection may occur upon bacteraemia seeding on a pre-existing injured intima or atherosclerotic plaque, infection of the aortic wall from an adjacent infected site, direct bacterial inoculation in case of penetrating injury, or septic emboli of the aortic vasa vasorum (generally in the setting of bacterial endocarditis) [5]. In this patient, the most likely cause of aortic infection responsible for the mycotic aneurysm was a septic embolus. This is supported by the fact that the patient was an intravenous drug user, with alcoholic habits and chronic liver disease (with an associated immunosuppressed state). All of these are risk factors for developing mycotic aneurysms, together with diabetes, chemotherapy and malignancy [5].
Septic emboli of the thoracic aorta usually occur in a setting of infective endocarditis [5]. Due to the absence of the most frequent clinical signs of endocarditis, such as fever, weight loss, anorexia, cardiac murmurs, skin manifestations or neurological symptoms, this hypothesis was not considered. However, we retrospectively consider that an echocardiogram could have been done to rule out this hypothesis.
In conclusion, aortoesophageal fistula caused by mycotic aneurysm rupture is rare and often fatal. A high level of suspicion is needed to prompt a fast diagnosis and intervention, and in this case, the EGD and CT results were crucial. However, we reason that an important clinical sign such as mid-thoracic pain cannot be disregarded in a patient aged 41 years with multiple comorbidities, even in the setting of a normal electrocardiogram and chest x-ray, to prevent a fatal outcome.