ABSTRACT
Tumor necrosis factor (TNF) receptor-associated periodic syndrome (TRAPS) is a rare hereditary systemic autoinflammatory disease (SAID). Treatment is based on corticosteroids, but often requires the addition of a biologic drug (anti-TNF agent, IL-1 receptor antagonist, etc) to achieve symptom control. The addition of the second drug is not clearly defined and must take into account the characteristics and preferences of the patient. We describe a patient with TRAPS and an allergic reaction to anakinra which was difficult to manage clinically while alternative treatment was being identified.
LEARNING POINTS
- Treatment of tumor necrosis factor (TNF) receptor-associated periodic syndrome (TRAPS) often requires adding a biologic drug to corticosteroids to achieve the best efficacy.
- Currently, IL-1 receptor antagonists are considered the first line of treatment in TRAPS.
- The most frequent adverse effect of anakinra is a reaction at the injection site.
- Canakinumab has shown better response compared to placebo in the treatment of TRAPS.
KEYWORDS
TRAPS syndrome, anakinra, allergic reaction, canakinumab
INTRODUCTION
Tumor necrosis factor (TNF) receptor-associated periodic syndrome (TRAPS) is an autosomal dominant autoinflammatory disorder caused by mutations in the gene for p55 TNF receptor type I (TNFR1) [1, 2]. TRAPS is one of the hereditary systemic autoinflammatory diseases (SAIDs) and is characterized by recurrent episodes of fever, arthralgia, periorbital oedema, myalgia, abdominal pain, serositis and skin rash [2]. Inflammatory acute episodes usually respond to oral corticosteroids, but often require increasing doses, especially in patients with frequent relapses or continuous symptoms [2, 3].
It is often necessary to add a biologic drug to the corticosteroids to increase response. One such drug is etanercept, an inhibitor of tumor necrosis factor-alpha (TNF-α), which decreases the frequency and/or intensity of attacks, allowing for a reduction in corticosteroid administration [2].
However, a decrease in responsiveness to etanercept may occur over time due to a non-specific action in TRAPS. In addition, long-term adherence to etanercept is poor and a significant number of patients need to switch to other biologic therapy [1, 2]. Over the last decade, IL-1 receptor antagonists have emerged as the first-line treatment option for TRAPS patients due to a significantly better clinical and biochemical response rate to treatment with anti-TNF [3, 4].
Anakinra, a recombinant IL-1 receptor that competitively inhibits the binding of IL-1-α and IL-1-β to its receptor, has shown promising results in patients resistant to etanercept. It has recently been shown that anakinra prevents relapse in both the short- and the long-term and induces rapid and stable remission. However, anakinra refractoriness has also been described and it has the disadvantage of needing daily subcutaneous administration with frequent skin reactions [2, 3]. Long-acting drugs targeting IL-1, such as canakinumab, a human anti-IL-1β, have also shown efficacy for the treatment of TRAPS [4]. In addition, canakinumab is easier for the patient since it is administered every 4–6 weeks. The choice of drug must be made individually taking into account the characteristics and preferences of the patient, as well as the experience of the physician.
CASE PRESENTATION
The patient was a 34-year-old woman with sorbitol and fructose intolerance. She denied any drug, alcohol or tobacco misuse. Her medical history included knee surgery for villonodular chondral synovitis in November 2017.
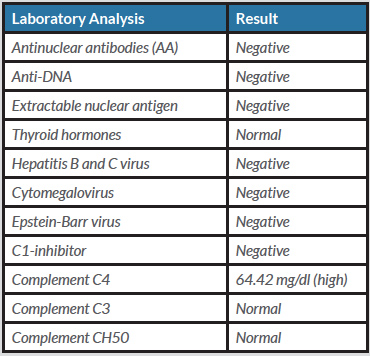
The patient had presented to the rheumatology department for the first time in June 2017, with arthromyalgia, oral thrush related to her food intolerance, recurrent tenosynovitis, a left scaphoid fracture after low-impact trauma, dizziness, a 1-month history of headache, and insomnia for approximately the previous year. She had no fever, skin rash or other associated symptoms. The results of previous medical tests were reviewed and revealed sustained elevation of the acute phase reactants. A complete autoimmunity study was requested (Table 1).
In July 2017, the patient developed daily afternoon low-grade fever. In September 2017, continuing low-grade fever, intense fatigue, a weight gain of 15 kg with no increase in usual food intake, dysphagia, alopecia and paraesthesia were noted.
In October 2017, the patient continued to present low-grade fever and paraesthesia, in addition to new generalized erythematous non-pruritic skin lesions and increased globular sedimentation rate (GSR) values. She presented arthritis in the wrist and dizziness. Electroneurography was then performed, with normal results. Blood tests showed normal CPK, aldolase 8.7 U/l and normal thyroid hormones.
In December 2017, the patient underwent genetic testing which revealed a heterozygous mutation at codon 92 (exon 4) of the TNFRSF1A gene consisting of a G>A change and predicting a change of arginine to glutamine in the protein (p.R92Q). In addition, it showed another heterozygous mutation in exon 3 of the NLRP12 gene consisting of a C>T change and predicting a substitution of histidine by tyrosine at the 304 position in the protein (p.H304T). Both mutations, although of low penetrance, are associated with autoinflammatory diseases compatible with TRAPS.
Upon diagnosis of TRAPS, the patient began treatment with oral corticosteroids. On February 15, as her symptoms had not improved, it was decided to start treatment with anakinra 100 mg daily. After starting the drug, the patient had a favourable evolution with remission of symptoms. However, on February 25, she presented to the emergency service approximately 10 hours after the ninth subcutaneous injection of anakinra, with well-demarcated, itchy and erythematous plaques at the injection sites. She started treatment with anti-H1.
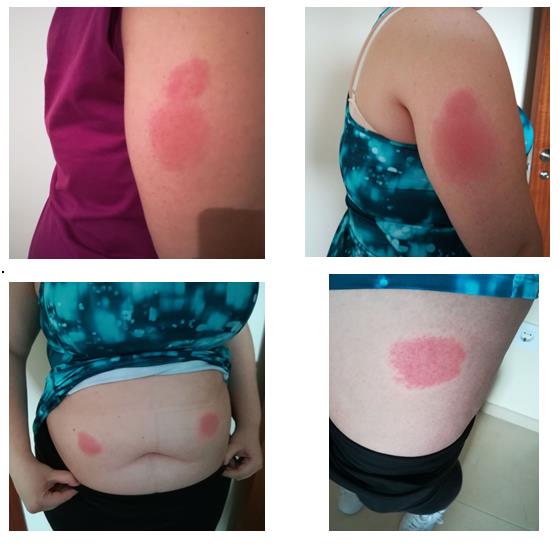
Two days later, the patient informed her rheumatologist that she had had to go to the emergency services because of a skin lesion on the left side of her abdomen which, a few hours later, had spread to the sites of previous injections (Fig. 1). On examination, the erythema felt hot, with a burning and itching sensation. The patient also presented fever with odynophagia and plaques in the pharynx in the last 72 hours. Recurrent fever had subsided with medication administered in the emergency service. It was decided to temporarily suspend treatment with anakinra and refer the patient for an allergology consultation.
In March 2018, after assessment by the allergology service, allergy to anakinra was confirmed and treatment was suspended. Canakinumab was then requested and approved by the rheumatology committee. Taking into account the patient's history and the risk of possible cross-reactivity to the drug, the pharmacy service prepared a 10 mg/0.2 ml aliquot of canakinumab in a horizontal laminar flow hood for allergy testing in the patient. After confirming good tolerance, treatment with canakinumab 150 mg was started in April, and administered every 8 weeks (instead of every 4 as indicated in the data sheet) under the supervision of the allergology service, together with 10 mg of prednisone.
In June, the second dose of canakinumab was administered without incident, so it was decided to discontinue corticosteroids. In November, the dosage was changed to 150 mg per month since the patient presented joint pain in the days just before administration of the next dose. At her last visit, the patient remained afebrile, was losing weight and her arthromyalgia was improving.
DISCUSSION
Our patient was diagnosed with TRAPS, which is a rare disease [5]. She began corticosteroid treatment but since disease symptoms persisted, the addition of a second drug was deemed necessary. As mentioned above, some studies have shown better results with anti-IL-1 compared with anti-TNF-α for the treatment of TRAPS [3, 4], so it was decided to replace etanercept with off-label anakinra.
Anakinra is administered daily and is usually well tolerated. The most frequent adverse effect is a reaction at the injection site [6], which has been reported in 50–80% of patients, requiring withdrawal of the drug in approximately 5% of cases [7]. Nevertheless, as stated on the drug´s data sheet [8], the rash is classified as a rare adverse effect. In addition, Olsen et al. [9] demonstrated in their study that only a few patients present more severe allergic reactions, including itching, swelling and pain.
A literature search revealed very few published cases of skin reactions to anakinra. Nor were there any studies on this adverse effect in TRAPS, probably because of the low prevalence of the disease. One of the articles identified [6] included five women with rheumatoid arthritis who had an anakinra-associated skin reaction. As in our patient, all of them presented skin lesions several days after the start of treatment (8–16 days) and two of them had to permanently suspend it.
In our case, the patient experienced symptom improvement after starting treatment with anti-H1. However, it was permanently discontinued after the allergology department confirmed that the drug was the main cause of the rash. In this situation, due to the lack of evidence concerning the efficacy of etanercept in TRAPS [1, 2], it was decided to change treatment to canakinumab.
Although canakinumab cannot be evaluated against other available biological therapies as no trials have compared the efficacy and/or safety of these drugs, a pivotal phase III study of canakinumab in patients with TRAPS [10] demonstrated a statistically significant better response rate compared with placebo (45% vs 8%). Furthermore, good tolerance with an acceptable safety profile was evident since the vast majority of adverse reactions were mild to moderate, thus suggesting a specific indication for TRAPS.
Our case is a unique example of a patient with TRAPS and an allergic reaction to anakinra, where the participation of different specialties in our hospital resulted in the initiation of new treatment.