ABSTRACT
Acquired haemophilia A (AHA) is a rare autoimmune disorder caused by an autoantibody against any circulating coagulation factor, especially factor VIII (FVIII). The lack of awareness of this condition suggests that diagnosis is a challenge and usually delayed, which leads to suboptimal treatment. Consequently, early diagnosis is mandatory to prevent potentially life-threatening bleeding complications. We present the case of an 85-year-old woman admitted to hospital with symptoms of respiratory infection who 12 hours later developed haematuria which required transfusion. Laboratory assays showed an isolated prolonged aPTT, a moderately reduced FVIII and a high inhibitor titre. Influenza A and Escherichia coli were also identified. Antivirals, antibiotics, immunosuppressive drugs and haemostatic agents were started. Two weeks later, the inhibitor was not detected, and bleeding and symptoms of infection had resolved. Immunosuppressive drugs were stopped on day 45 and there has been no recurrence since then. To date, no FVIII inhibitors have been reported in concomitant infection with influenza A and urinary E. coli. The identification of conditions potentially associated with AHA is essential to achieve complete remission.
LEARNING POINTS
- The lack of awareness of and experience with acquired haemophilia (AHA) suggests that diagnosis is frequently delayed, resulting in suboptimal treatment.
- AHA should be suspected in recent-onset abnormal bleeding in patients not receiving anticoagulant treatment, and in the presence of isolated prolonged activated partial thromboplastin time (aPTT).
- Treatment is based on eradication of the inhibitor, control of the bleeding and identification of underlying conditions.
KEYWORDS
Acquired haemophilia, bleeding disorders, influenza, infectious diseases
INTRODUCTION
Acquired haemophilia A (AHA) is a rare bleeding disorder caused by an autoantibody against any circulating coagulation factor, especially factor VIII (FVIII). The so-called inhibitor interferes with FVIII cofactor function, thus hindering thrombin production. Incidence is estimated to be about 1.5 cases per million population per year [1]. There is a higher rate of AHA in people over 68 years of age, especially men, while pregnant women account for most cases in people below the age of 40 [1]. AHA cases have been described in the context of other pathologies and conditions such as autoimmune disease, malignancy, infection, pregnancy and medication intake. Nevertheless, up to 50% of reported cases are idiopathic, and the mechanisms predisposing to inhibitor onset remain poorly understood [1]. Early diagnosis is mandatory to prevent potentially life-threatening complications [1]. We present the case of an elderly woman who presented with AHA in the context of a complex infectious scenario consisting of influenza A (H1N1) and Escherichia coli-mediated urinary tract infection (UTI). The condition requires prompt diagnosis to prevent complications in patients who are often in a fragile condition.
CASE DESCRIPTION
We present the case of an 85-year-old woman who attended the emergency department with a 1-week history of dyspnoea, cough, and fever (>39ºC). Her medical history included arterial hypertension, right kidney agenesia, moderate chronic kidney disease (CKD), arthrosis and peripheral venous insufficiency. She had no previous history of a bleeding disorder. At hospital arrival, she was taking omeprazole, tapentadol, pregabalin, eprosartan, hydrochlorothiazide and calcium as chronic medication, and oxacillin after a fall 2 weeks previously causing abrasion of the lateral side of the left knee as well as bruises on her face and lower limbs.
Pulmonary and cardiac auscultation revealed generalized rhonchi and crackles, and multifocal atrial arrhythmia with arrhythmic heart sounds at 110 bpm with no murmurs. The main blood test findings were: (a) blood cell counts: haemoglobin (Hb) 8.4 g/dl, leucocytes 13.24×103/μl and neutrophils 10.97×10³/μl, and normal platelet (P) counts 377×103/μl; (b) biochemical parameters: creatinine clearance (CrCl) 30 ml/min/1.73 m2, and normal hepatic function and ionogram; (c) coagulation parameters: prothrombin time (PT) 84% (normal range (NR) 70–120%), INR 1.12 (NR <1.3), activated partial thromboplastin time (aPTT) 74.3 sec (NR <40 sec) and fibrinogen 613 mg/dl (NR 150–400 mg/dl), and (d) inflammatory parameters: elevated C-reactive protein 22.79 mg/dl (NR 0–5 mg/dl) and procalcitonin 23.64 ng/ml (NR <1 ng/ml). At hospital admission, the chest x-ray was normal, urine and blood cultures were obtained, and respiratory virus testing was performed. Combination antibiotic therapy with piperacillin/tazobactam (due to elevated procalcitonin) and oseltamivir (because of a positive result for H1N1 influenza A) was started. A single prophylactic dose of enoxaparin (40 mg) was also administered. However, 12 hours later, the patient showed macroscopic haematuria and haematomas appeared around venipuncture sites and Hb dropped to 6.4 g/dl, requiring up to 6 red blood cell units. Isolated aPTT remained prolonged at 94 sec. A coagulation disorder was therefore suspected.
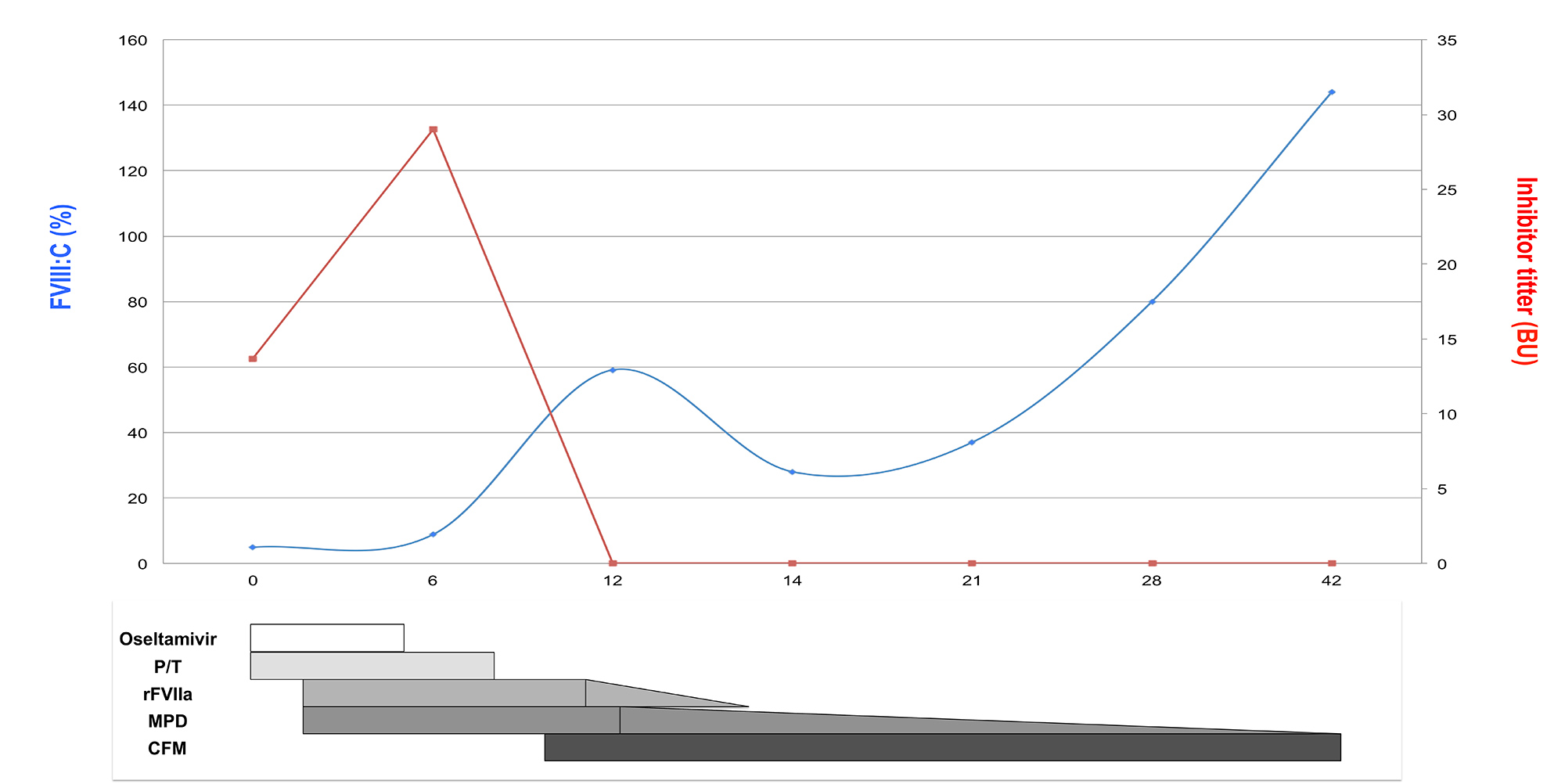
The patient’s plasma was mixed with normal pooled plasma in a ratio of 1:1 and incubated, which partially corrected the aPTT, reducing it to 66.6 sec [1]. Further investigation revealed FVIII activity of 5% (NR 70–150%) and antifactor VIII inhibitor antibody levels of 13.65 Bethesda units (BU) per ml (NR <0.5 BU/ml) measured according to standard recommendations [1]. Von Willebrand syndrome was ruled out. Following AHA diagnosis, the patient was treated for 9 days with recombinant activated factor VII (rFVIIa) at a dose of 90 μ/kg/4 h to control the active bleeding (Fig. 1). Due to active infection, eradication inhibitor treatment was started with low-dose corticosteroids (methylprednisolone (MPD), 0.5 mg/kg/day). After 9 days, cultures were negative and antibiotic and antiviral therapy were stopped, but levels of inhibitor antibodies rose again to 29 BU/ml, so cyclophosphamide (50 mg/day) was added (Fig. 1). After 12 days, the inhibitor was eradicated, FVIII activity increased to 59% and bleeding completely resolved. Computed tomography (CT), serology for hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV), immunoglobulin levels and proteinogram, and autoimmune biomarkers were all negative. The patient was discharged and immunosuppressive treatment finished on day 45. She has been in complete remission since.
Figure 1. Timeline of changes in FVIII activity and inhibitor titre related to haemostatic and immunosuppressive therapy.[Q9] BU, Bethesda units; CFM, cyclophosphamide; FVIII:C, factor VIII activity; MPD, methylprednisolone; P/T, piperacillin/tazobactam, rFVIIa, recombinant activated factor VII
DISCUSSION
FVIII inhibitors are a rare cause of coagulopathy. Diagnosis is challenging and if delayed can lead to serious outcomes, which are frequently worsened by the age and poor condition of the patient [1, 2]. It is therefore important to identify any underlying disease that may increase the risk of inhibitor development so that AHA can be diagnosed or ruled out. Our patient presented with a FVIII inhibitor in the context of respiratory and urinary infection.
The fast diagnosis of AHA and the concomitant infection allowed us to rapidly control the bleeding with rFVIIa, immunosuppressive drugs and supportive care (Fig. 1) [1–3]. The actions taken to eradicate the inhibitor were also determined by the patient´s fragility and the need to avoid excessive immunosuppression. MPD was started immediately after AHA diagnosis using doses lower than normally recommended[1,2]. Furthermore, cyclophosphamide was not started until infections had been controlled. Nevertheless, response was very good, with the patient achieving complete remission in 12 days, with no subsequent recurrence (Fig. 1).
The identification of underlying conditions predisposing to inhibitor development allows fast recognition of AHA. As anticipated, no underlying cause is found in about 50% of cases, particularly in elderly patients [1, 3]. Indeed, old age may be an independent risk factor, but the possibility of associations with diseases other than neoplasia or autoimmune disorders remains to be investigated. Our patient presented with the inhibitor in a complex scenario consisting of influenza A and E. coli infection, as well as chronic intake of neuropathic, antihypertensive and opioid analgesic drugs, and oxacillin consumption for 4 days. Beta-lactam antibiotics have been associated with AHA, since they are widely used by the elderly, but the number of reported cases is small and it is not clear whether they are causative or coincidental [1, 3, 4].
Interestingly, to our knowledge no cases of AHA associated with influenza A and/or E. coli urinary infection have been reported to date. A review focused on the elderly population found no associations between underlying infectious processes and AHA in 159 patients aged 65 years or over [3]. Isolated cases of AHA have been described in the context of patients with human herpesvirus 8, HIV, HCV, HBC or parvovirus B19 infection, some of whom were being treated with interferon [1, 3, 5, 6]. Influenza viruses have an array of strategies to disarm the host immune system [7], and thus they may be candidates for prompting FVIII inhibitor development. On the other hand, AHA subsequent to penicillin-treated upper respiratory tract [8], ear [9] or paediatric streptococcal infections [10], but not urinary infection, has also been documented.
Our work has some limitations. The double infection with influenza A and E. coli and the intake of oxacillin precludes the establishment of direct associations between such conditions and the inhibitor. Nevertheless, our case is unique in that no association between AHA and influenza A and/or E. coli infection has been described so far.
CONCLUSIONS
Suspicion of and prompt diagnosis of AHA is essential so that successful haemostatic and inhibitor eradication therapies can be implemented. Identification of clinical conditions associated with AHA may be useful for prompting inhibitor search as early as possible. The fragile condition of patients mandates the use of conservative treatment.