ABSTRACT
Subclavian vein access is still one of the most favoured access options for cardiac implantable electronic device (CIED) implantation. For the physician, the technique is reasonably familiar and easy to carry out. However, this has several potential complications. In this case, we present a late complication of subclavian access. The patient presented with intermittent loss of pacemaker output, which caused him to experience several syncopal events. In the acute setting, we changed the lead polarity and achieved a good outcome. Further management of this situation consisted of removal and replacement of the damaged lead.
LEARNING POINTS
- Subclavian vein access is still one of the most favoured access options for cardiac device implantation.
- Intermittent loss of output can be a sign of pacemaker malfunction due to subclavian crush syndrome.
- Damaged lead extraction may be difficult to perform.
KEYWORDS
Pacemaker malfunction, subclavian crush syndrome
INTRODUCTION
Cardiac implantable electronic devices (CIEDs) are among the most commonly implanted medical devices currently [1]. They are used to treat heart rhythm problems such as symptomatic bradycardia (cardiac pacemaker), life-threatening arrhythmia (intracardiac cardioverter-defibrillator; ICD) or heart failure (cardiac resynchronization therapy; CRT) [2]. There are two main components, the pulse generator and one or more intracardiac leads. There are several choices available for vein access. Subclavian vein puncture is still one of the preferred options, and was the first choice for vein access in 40% of the European centres participating in the European Heart Rhythm Association survey [3].
Physicians are relatively familiar with the subclavian access technique and require minimal surgical skill [4]. However, this has a slightly higher rate of potential complications. In an acute setting, subclavian access was associated with a higher rate of pneumothorax [5]. Later after implantation, it can also cause pacemaker malfunction due to subclavian crush syndrome [4]. Here, we report a case of intermittent pacemaker malfunction caused by subclavian crush syndrome.
CASE DESCRIPTION
A 69-year-old male presented to the emergency room of his local area hospital. The patient had been suffering from recurrent syncopal events for 3 days prior to admission. From his previous medical history, he had undergone pacemaker (Medtronic Sensia SR/single chamber) implantation one year previously due to degenerative complete atrioventricular block. The patient was lost to follow-up for approximately 8 months.
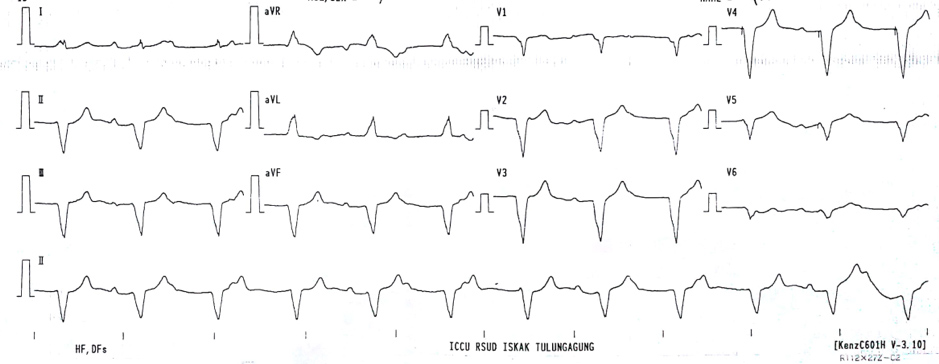
During admission, the patient was haemodynamically stable. The emergency physician performed an initial electrocardiogram that showed pacemaker rhythm (VVI) with a heart rate of 60 bpm (Fig. 1). There was no sign of pacemaker malfunction. The patient was then transferred to the general ward for further evaluation of the cause of syncope. Pacemaker interrogation was not performed at that time as the programming device was not available in that hospital.
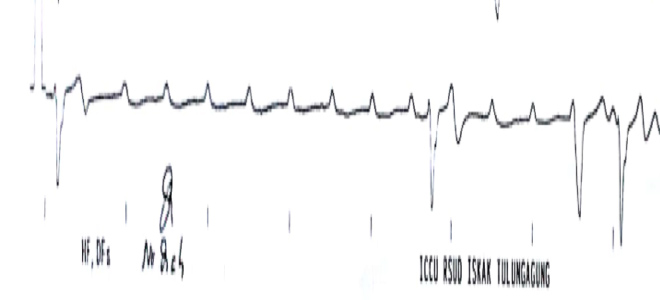
In the early hours of observation, nothing untoward occurred. Blood work was concluded as normal and no abnormalities were found. However, another syncopal attack took place, followed by cardiac arrest. The patient underwent resuscitation. The ECG at that time showed an episode of ventricular asystole without any sign of a pacemaker spike on ECG strips (Fig. 2). The patient was then transferred to the catheterization laboratory for transvenous temporary pacemaker insertion. During the procedure, the attending physician noticed that the permanent pacemaker lead was in the correct position (right ventricular apex) but there was a noticeable breakage in the lead continuity.
Figure 1. Initial ECG obtained in the emergency room after the patient had recovered from syncope, showing normal ventricular pacing rhythm
Figure 2. An ECG strip recorded during the syncopal event showed prolonged ventricular asystole. A pacing spike was not found
The following day, the patient was transferred to our hospital. The pacemaker interrogation revealed high impedance (1.780 ohms) suggesting a lead fracture, with a high pacing threshold. We attempted to change the lead polarity from bipolar to unipolar, which resulted in better impedance and a normal pacing threshold. We also decided to carry out lead replacement for this patient.
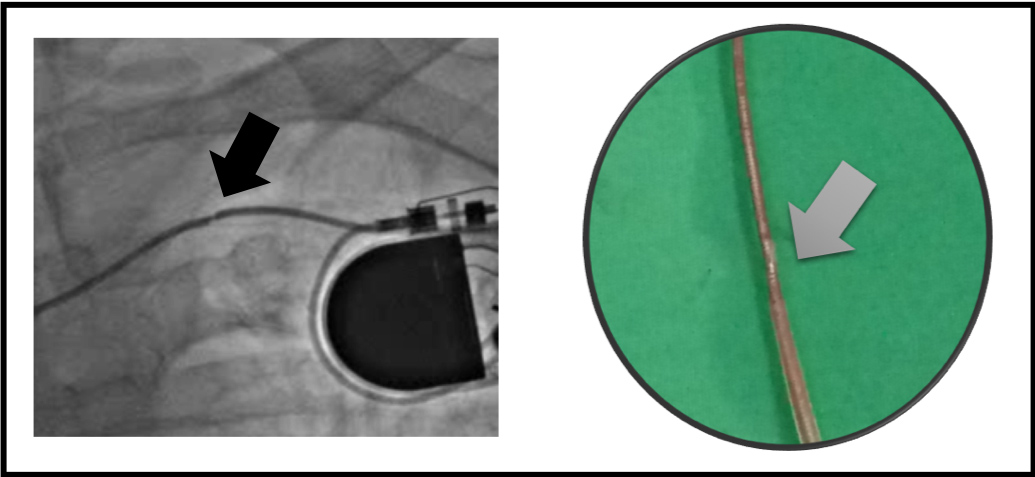
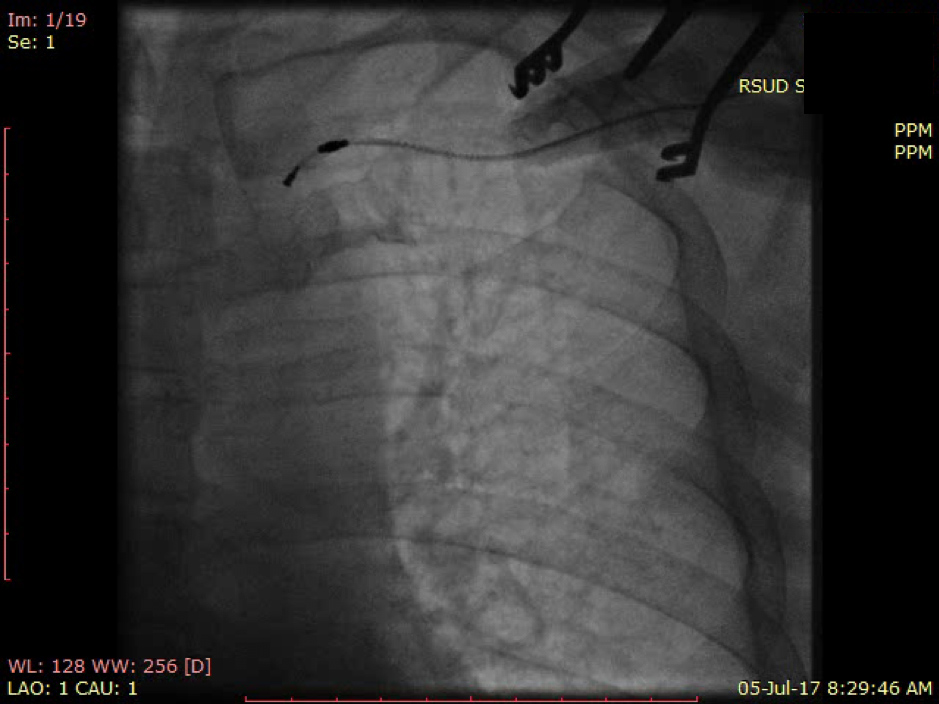
During the procedure in the catheterization laboratory, we attempted to retract the broken lead. It was an active-fixatioN lead so relatively easy to pull out from the right ventricular apex after we unscrewed it (Fig. 3). However, when the head of the pacing lead reached the subclavian area, it became trapped. Despite employing several manoeuvres, we still could not pull the head of the pacing lead past the area between the first rib and clavicle (Fig. 4). Therefore, we decided to cut this out and insert a new lead using the cephalic vein cut-down approach.
Figure 3. Left: Fluoroscopic image showing lead indentation in the subclavian region. Right: The extracted lead showed a lead fracture
Figure 4. The head of the pacing lead was trapped within the subclavian region and could not be extracted
DISCUSSION
Lead fractures are one of the delayed complications of CIED implantation. With respect to the choice of vein access, subclavian access is associated with a higher incidence of future lead fractures compared to cephalic and axillary vein access [6, 7]. Subclavian crush syndrome is the term used to describe lead fractures caused by continuous compression of the lead by the clavicle and first rib. It occurs more commonly with bigger lead sizes and when multiple leads are involved, such as with ICD and dual chamber pacemaker systems, compared to the single chamber pacemaker lead. Apart from the type of lead, there are patient risk factors associated with lead fractures.
Patients with a low BMI had a higher risk of lead fractures [8]. Lead engineering also plays an important role in lead longevity. Coaxial lead design, where each of the conductors is wrapped around the cathode coil conductor and separated by insulation, is more likely to result in fractures. Coaxial design was used in the early years of ICD development. Recent developments in lead design engineering have involved the use of the multilumen design [9]. In our patient, subclavian access was chosen for vein access and the patient’s BMI was less than 20 kg/m2.
The incidence of subclavian crush syndrome can also be predicted by several angiographic parameters, such as pocket, intravascular and intracardiac lead characteristics [10].
The clinical consequences of subclavian crush syndrome are wide-ranging. In cases of ICD lead fracture, inappropriate ICD shock, failure to detect malignant arrhythmia and failure to deliver appropriate treatment can occur [9]. A rare case has been reported where lead fractures resulted in pulmonary embolism caused by the lead debris. For cardiac pacemakers, subclavian crush syndrome can result in failure to deliver bradycardia therapy [8]. In other cases, it has been reported that subclavian crush syndrome can be asymptomatic and can be detected unintentionally during routine follow-up. It can manifest as high lead impedance or as lead polarity automatically changed to the unipolar setting. In our case, lead fractures caused symptomatic bradycardia and an episode of cardiac arrest. The pacemaker failed to deliver pacing while the patient was in a pacemaker-dependent condition.
Subclavian crush syndrome is mostly managed by extraction of the damaged lead and full replacement with a new one. The problem is, not all damaged leads can be extracted. Lead extraction is a complex procedure [11]. In our case, the lead could be pulled out from the right ventricular apex only by using a simple traction technique [12]. It was an active-fixation lead, and had just been implanted a year previously. According to the ELECTRa Registry, the simple traction technique has a high success rate, especially if implantation took place less than 2 years previously [13]. The problem occurred when the head of the pacing lead reached the area between the clavicle and the first rib. The head of the pacing lead could not pass by, even after several manoeuvres involving the patient’s deltoid area. Therefore, we decided to cut it out, and left the remaining material in situ.
CONCLUSION
The rate of acute or late complications associated with using subclavian access for the implantation of CIEDs is quite low. However, physicians need to be aware of possible complications. An additional syncopal event occurring after the implantation procedure should be initially investigated as a possible CIED malfunction. In this case, the loss of output caused several syncopal events. We were unable to remove the damaged material completely, so we decided to cut the lead and insert a new one.