ABSTRACT
Background: Variegate porphyria (VP) is a rare disorder of haem biosynthesis. We report a novel association with hepatitis A infection.
Patient and methods: A 31-year-old man was diagnosed with acute hepatitis A infection. During recovery, he presented with abdominal pain and a photoaggravated blistering skin eruption.
Results: Urine porphyrin precursors were markedly raised with high coproporphyrin III isomer levels. Faecal protoporphyrin levels were markedly increased and a maximum plasma fluorescence emission at 629 nm was noted.
Discussion:Acute hepatitis A infection, and the associated metabolic stress exerted on the haem biosynthetic pathway, induced overt presentation of latent VP.
LEARNING POINTS
- There should be a high index of suspicion for an acute cutaneous porphyria when a photosensitive rash is accompanied by neurovisceral symptoms.
- Latent porphyria may be overtly manifested after appropriate triggers which stress the metabolic haem biosynthetic pathway. One such trigger demonstrated by this case presentation is acute hepatitis A infection.
- The diagnostic approach to the investigation of a suspected acute cutaneous porphyria is initially with light-protected samples for urinary porphyrin precursors and plasma for fluorescence scanning. These should be sampled ideally during symptomatic periods. Further specialist analysis with fractionation of urinary and faecal porphyrins is necessary to distinguish between the two different acute cutaneous porphyrias.
KEYWORDS
Variegate porphyria, hepatitis A
CASE DESCRIPTION
A 31-year-old Finnish man, with HIV-seropositivity on abacavir, lamivudine and raltegravir, was admitted to hospital with fever, epigastric pain, nausea and vomiting. The biochemical liver profile was suggestive of acute hepatitis (ALT 822 IU/l, GGT 77 IU/l, ALP 94 IU/l, total bilirubin 147 µmol/l). Hepatitis serology revealed a positive hepatitis A IgM and the patient received supportive care for acute hepatitis A infection. He made an uneventful recovery, with his liver biochemical profile returning to normal within 3 weeks.
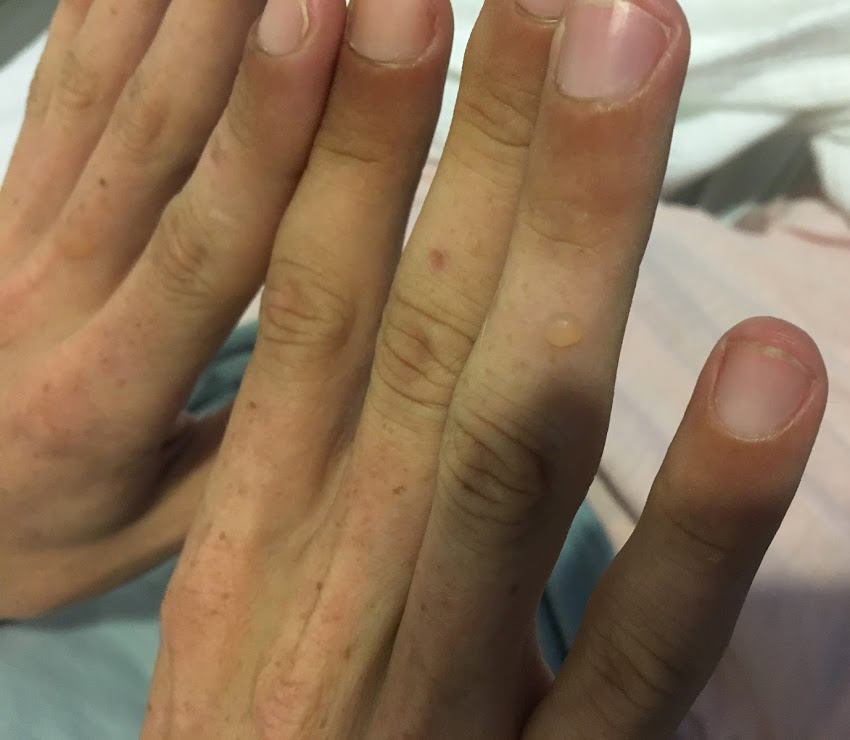
The patient was readmitted to hospital 1 week later with abdominal pain and a mild blistering eruption on the scalp (the patient shaves his hair), face, neck and the dorsal hands (Fig. 1) with minimal background erythema.
The eruption was pruritic, associated with a burning sensation, and symptoms were photoaggravated. A cutaneous porphyria was suspected; however, the results from a sample taken for free and zinc erythrocyte porphyrins were within reference intervals.
Figure 1. Photo showing a tense blister on the right ring finger of the patient
Methods and Procedures
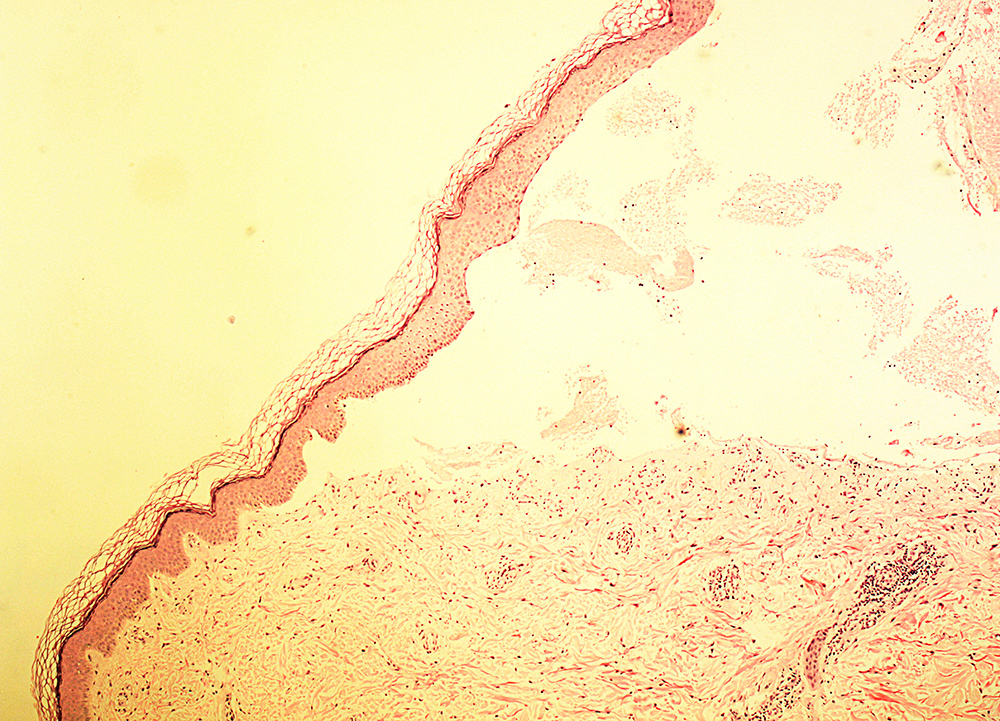
A skin biopsy (Fig. 2) showed a cell-poor subepidermal blister with festooning of the dermal papillae into the blister floor. There was only a sparse inflammatory cell infiltrate and the blister predominantly contained erythrocytes. Direct immunofluorescence revealed deposition of IgG and of C3 at the basement membrane zone of the epidermis as well as weak equivocal deposition around blood vessels. The findings were in keeping with, but not diagnostic of, porphyria. Histological differentials include pseudoporphyria, hereditary epidermolysis bullosa and cell-poor pemphigoid, but there were no other features to entertain these alternatives.
Figure 2. Skin biopsy (H&E) showing subepidermal, cell-poor bullae with festooning of the dermal papillae with sparse inflammatory cell infiltrate. These findings are characteristic but not specific for porphyrias
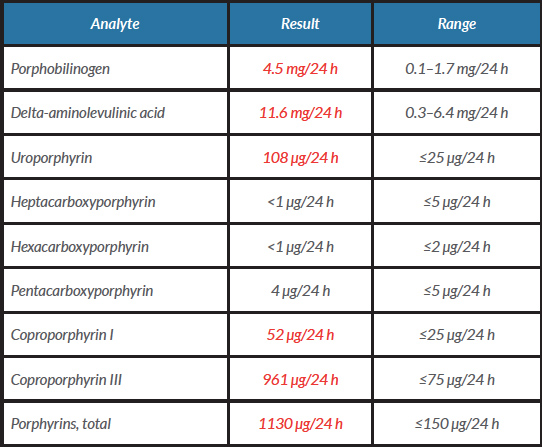
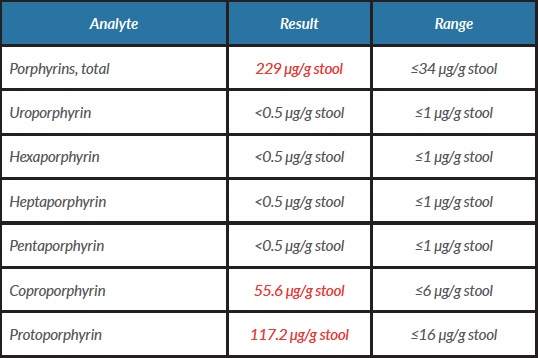
Further investigations were undertaken including light-protected sampling of urinary porphyrins and the porphyrin precursors porphobilinogen (PBG) and delta-aminolevulinic acid (ALA). The latter were both raised, together with abnormally elevated levels of urinary porphyrins, particularly coproporphyrins and uroporphyrin to a lesser extent. A stool specimen was also sent for porphyrin analysis. This showed raised total faecal porphyrins with both protoporphyrin and coproporphyrin being elevated. Plasma porphyrins were also increased with a fluorescence emission peak maximal at 629 nm. Taken together, these biochemical findings are diagnostic of variegate porphyria (Tables 1 and 2).The patient was thereafter advised regarding avoidance of sunlight and photoprotection, avoidance of precipitating factors, and vitamin D supplementation.
Table 1. Results of urinary porphyrins and the porphyrin precursors porphobilinogen (PBG) and delta-aminolevulinic acid (ALA) taken during a symptomatic period
Table 2. Results of stool porphyrin analysis
Values in red are outside the range
DISCUSSION
Hereditary porphyrias are a group of rare metabolic disorders resulting from enzymatic defects in the haem biosynthetic pathway. Acute porphyrias may present with neurovisceral attacks and are associated with elevations in the haem precursors PBG and ALA. Cutaneous porphyrias present with skin fragility and blistering in photoexposed areas or a burning sensation after sun exposure. Acute cutaneous porphyrias (variegate porphyria (VP) and hereditary coproporphyria (HCP)) may present with both cutaneous features and neurovisceral attacks [1].
Porphyrias may be precipitated by triggers which induce metabolic stress including infection, fasting, medications, alcohol or cyclical hormonal changes [2]. In the present case, the patient had a latent porphyria due to an inherited deficiency of protoporphyrinogen oxidase in the haem biosynthetic pathway. Overt manifestations were triggered following acute hepatitis A infection and the metabolic stress this caused on haem synthesis.
The biochemical basis for this is the induction of ALA synthase, the first irreversible and rate-limiting step in the biosynthesis of haem from succinyl CoA and glycine. The increase in ALA synthase activity leads to the overproduction of ALA, PBG and specific porphyrins up to the metabolic block. ALA and other metabolites which accumulate in acute porphyrias are neurotoxic and cause neurovisceral attacks. The accumulation of highly photoreactive porphyrins in the skin results in blisters without significant surrounding inflammation [1,2]. There is a well-established link between hepatitis C, HIV and porphyria cutanea tarda [2], however to our knowledge this is the first published case of an acute cutaneous porphyria triggered by acute hepatitis A infection. We assessed the patient’s anti-retroviral therapy combination and considered this non-porphyrogenic and safe for use with acute porphyria based on database searches.
A diagnosis of porphyria can be challenging and is often delayed by years. Porphyrias should be considered when patients present with cutaneous features such as photodistributed blistering eruptions which are otherwise relatively uninflamed, milia or hypertrichosis, with or without systemic symptoms such as abdominal pain, neuropsychiatric features, autonomic instability, and hyponatraemia [1,3]. First-line investigations should be directed by the clinical presentation. In a suspected acute porphyria, light-protected urinary PBG and ALA are essential, while in patients presenting with cutaneous porphyrias, testing for a plasma fluorescence emission peak is a first-line test, except for rare cases of patients with acute painful photosensitivity who would require erythrocyte porphyrins [1, 3]. A skin biopsy or erythrocyte porphyrins (which were initially performed in our case) should not in fact be first-line investigations, and indeed, erythrocyte porphyrins were negative and could have been misleading. Further investigations including urinary and stool porphyrins are often required based on the results of the first-line tests [1–3]. In our case, the latter were performed first because plasma fluorescence was not readily available. DNA studies are helpful to identify a proband in the index patient and for screening family members for latent porphyria to provide advice on the list of possible safe drugs and the prevention of acute attacks [1–3].
In our case, an acute cutaneous porphyria was suspected and differential diagnoses include VP and HCP. The former, however was favoured based on the markedly raised coproporphyrin CIII isomer levels in urine and the raised stool protoporphyrin. Plasma fluorescence scanning discriminates between VP and HCP based on different emission peaks and in VP, plasma porphyrin fluorescence remains abnormal for many years following remission of symptoms [1].
The treatment of acute and cutaneous porphyrias differs. Attacks of the acute porphyrias should be treated urgently with agents inhibiting ALAS-1 activity (intravenous glucose and haemin), while at the same time avoiding potential triggers. For patients with skin blistering, avoidance of sunlight is important, thus patients should also receive vitamin D supplementation. Application of a special high sun protective factor reflectant sunscreen with protection from the visible light and ultraviolet spectra is recommended as well as the use of protective tinted glass for windows and cars [1, 4]. In acute porphyrias, drug safety issues need to be taken into account since drugs metabolised by the cytochrome P450 enzyme pathway may also trigger symptoms [1, 4].
This case demonstrates the non-specific nature porphyria could present with. Abdominal pain could easily have been dismissed as related to hepatitis and there are several causes of blistering disorders. This shows that despite being rare, a diagnosis of porphyria should be included in the differential diagnosis when appropriate since this has potentially life-saving implications for the patient and their relatives.