ABSTRACT
In December 2019, a novel coronavirus called SARS-CoV-2 was reported to be responsible for a cluster of acute atypical respiratory pneumonia cases in Wuhan, in Hubei province, China. The disease caused by this virus is called COVID-19 (coronavirus disease 2019). The virus is transmitted between humans and the outbreak was declared a pandemic by the World Health Organization (WHO) on 11 March 2020. Coagulopathy is a common abnormality in patients with COVID‐19 due to inflammation, hypoxia, immobilisation, endothelial damage and diffuse intravascular coagulation. However, the data on this topic are still limited. Here we report the case of a man presenting with pneumonia complicated by bilateral pulmonary embolism
LEARNING POINTS
- SARS-CoV-2 is a novel infectious agent that causes COVID-19, which can manifest in several ways, affecting endothelial cells and most organs.
- There is growing evidence that SARS-CoV-2-mediated endothelial damage is due to direct viral injury and the systemic inflammatory response, possibly together with a cytokine storm.
- As endothelial damage can manifest as thromboembolic disease, such as pulmonary thromboembolism, appropriate anti-thrombotic preventive strategies should be followed, and proper screening and treatment for thromboembolic complications should be implemented.
KEYWORDS
Coronavirus, COVID-19, SARS-CoV-2, pulmonary embolism, coagulation abnormalities, tocilizumab
INTRODUCTION
Coronaviruses are enveloped non-segmented positive-sense RNA viruses, and since the previous epidemics of SARS-CoV and MERS, are considered to be the major pathogens causing atypical pneumonia [1]. COVID-19, caused by SARS-CoV-2, first appeared in December 2019 in Wuhan, China and has since rapidly spread around the world. On 11 March 2020 it was declared a public health emergency by the World Health Organization [2, 3]. Common symptoms range from constitutional and upper respiratory problems to severe olfactory and gustatory dysfunction [4-6]. A hypercoagulable state is a common abnormality in patients with COVID‐19, and is due to infection, inflammation, hypoxia, immobilisation, and diffuse intravascular coagulation with marked elevations seen in lactate dehydrogenase, ferritin, C-reactive protein, D-dimer and interleukin levels [7,8]. Concomitant pulmonary embolisms have been detected on the CT scans of patients hospitalized mainly for respiratory symptoms due to COVID-19 [9,10]. Despite the growing literature on COVID-19 and coagulation abnormalities, data are limited and quality studies are needed. Here we describe a case of COVID-19 infection complicated by pulmonary embolism.
CASE DESCRIPTION
On 21 March 2020, a 50-year-old Caucasian man was referred to the emergency department with a 2-day history of fever and sore throat. He also had constitutional symptoms including myalgia, rhinorrhoea, cough and headache. His medical history was otherwise significant for chronic kidney disease, hypertension and hepatitis B (HBV). He was being treated with candesartan/hydrochlorothiazide and entecavir.
On physical examination, the patient appeared pale and sick. Vital signs were significant for a body temperature of 38.5°C, oxygen saturation of 95% on room air and a respiratory rate of 20/min. Lung sounds were decreased at the bases, with right lower-lobe rales. Chest radiography showed bilateral opacities with a peripheral distribution highly suggestive for interstitial pneumonia. A rapid test for influenza A, B and respiratory syncytial virus was negative. Urinary antigen tests for Streptococcus pneumoniae and serotype 1 Legionella pneumophila were negative. A nasopharyngeal and oral swab for SARS-CoV-2 was positive. Arterial blood gas analysis in ambient air confirmed type 1 respiratory failure (PaO2 9.1 kPa), associated with respiratory alkalosis (pH 7.46 and PaCO2 4.02 kPa). Blood analysis showed a normal leucocyte count with mild lymphocytopenia of 1180/µl, C-reactive protein (CRP) of 55 mg/l and acute kidney failure KDIGO stage 1. Treatment with lopinavir/ritonavir and hydroxychloroquine for 7 days was initiated. In addition, we prescribed parenteral physiological saline and anti-thrombotic prophylaxis with enoxaparin.
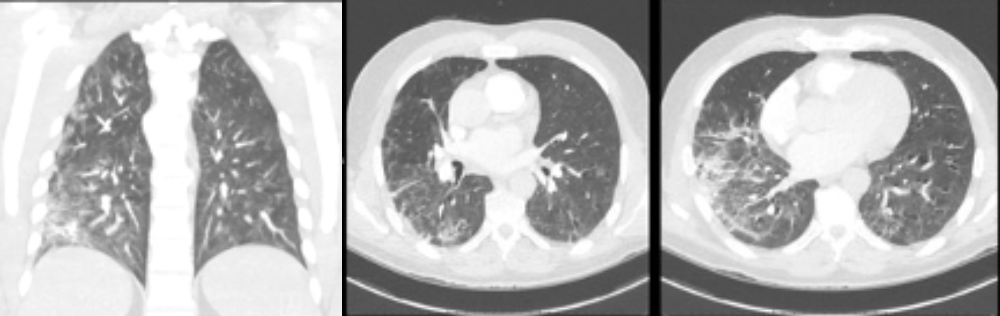
Nevertheless, the patient’s clinical status progressively worsened during 5 days of hospitalization: he developed dyspnoea and oxygen saturation dropped to 85% on room air. A thoracic CT scan (Fig. 1) showed bilateral multifocal ground-glass opacities, predominant in the inferior lobes, with confluent lesions. High-flow oxygen therapy was administered. Blood tests showed a normal leucocyte count with lymphocytopenia of 870/µl, and worsening of CRP up to 174 mg/l. On 26 March 2020, a single dose of tocilizumab 800 mg was administered. During the following days, the patient’s clinical status progressively improved and he was weaned from oxygen therapy.
Figure 1. Pulmonary CT images reveal multifocal bilateral ground-glass opacities with consolidation and a peripheral pattern, suggestive of SARS-CoV-2 infection
However, on 5 April 2020, before he could be discharged, the patient’s clinical status deteriorated again. He complained of palpitations, shortness of breath and chest pain, and oxygen saturation dropped to 90%. The ECG showed a tachycardic sinus rhythm and negative T waves in lateral derivations, which were not previously present. Blood tests showed high sensitivity cardiac troponin (hs Tc) in the normal range but D-dimer elevated at 2.7 mg/l. In light of the symptoms and ECG alterations, coronary angiography was performed, but was negative. A pulmonary angiography CT scan documented the presence of a bilateral filling defect diagnostic for pulmonary embolism (Fig. 2), and associated with extensive ground-glass opacifications involving both lung parenchyma with predominant consolidation in the posterior basal segment of the right lower lobe. Lower-limb compression ultrasonography was negative. Transthoracic echocardiography (TTE) was normal. Based on these findings, treatment with enoxaparin was started and the patient was closely monitored. He remained haemodynamically stable and was transitioned to oral anticoagulant therapy with rivaroxaban. On 14 April 2020, the patient was discharged in good condition.
Figure 2. Pulmonary angiography CT demonstrates multiple peripheral bilateral filling defects involving tributary branches of the pulmonary artery located on the basal pulmonary pyramid
DISCUSSION
Case reports, case series and articles in the literature have shown an association between COVID-19 and venous thromboembolic disease [11–12]. A pathophysiological explanation is that it could be due to both the systemic inflammatory response, possibly together with a cytokine storm, and direct endothelial damage [13-14]. Direct endothelial injury could be the characteristic feature as with other infectious agents. In fact, viral elements of SARS-CoV-2 have been described within endothelial cells, causing apoptosis and pyroptosis.
Due to these preliminary findings, in our institution an increased dose of thromboprophylactic heparin has been approved for patients with COVID-19, when feasible. Thus, in patients infected by SARS-CoV-2 it is extremely important to consider vascular complications as a possible cause of clinical deterioration. Overlooking thromboembolic phenomena could lead to a poor outcome and could partially explain the poor survival rates described in critically ill patients. Another therapeutic strategy could be to stabilize the endothelium while tackling viral replication with anti-inflammatory anti-cytokine drugs, ACE inhibitors and statins [15, 16]. Nevertheless, more studies are required to identify the best preventive and therapeutic approaches.
In conclusion, we present a case report of a COVID-19 patient, whose clinical course was complicated by bilateral pulmonary embolism. In patients affected by SARS-CoV-2 infection, particular attention should therefore be paid to possible vascular complications in order to prevent clinical deterioration and death.