ABSTRACT
The lifetime prevalence of peptic ulcer disease (PUD) is 5–10%. While PUD in immunocompetent patients is most commonly associated with Helicobacter pylori infection or the use of non-steroidal anti-inflammatory drugs (NSAIDs), other common causes of PUD must also be considered in the differential diagnosis. We describe a case of endoscopic and histological resolution of PUD related to Candida infection in a healthy, immunocompetent woman.
LEARNING POINTS
- Peptic ulcer disease (PUD) can be secondary to fungal infections, even in immunocompetent patients.
- A higher index of suspicion needs to be maintained for fungal causes of PUD, particularly if symptoms do not improve.
- Recognizing fungal causes of PUD may lead to faster diagnosis and treatment.
KEYWORDS
Hypokalaemia, rhabdomyolysis, liquorice abuse
CASE DESCRIPTION
A 50-year-old woman presented with a 3-month history of post-prandial abdominal cramping, vomiting and a 7 lb weight loss. Her medical history was significant for hypertension, hyperlipidaemia, depression, chronic obstructive pulmonary disease (COPD) and gastroesophageal reflux disease. There was no history of HIV (human immunodeficiency virus) or diabetes. She denied non-steroidal anti-inflammatory drug (NSAID) use and alcohol consumption and was not taking corticosteroids. Endoscopic evaluation was performed.
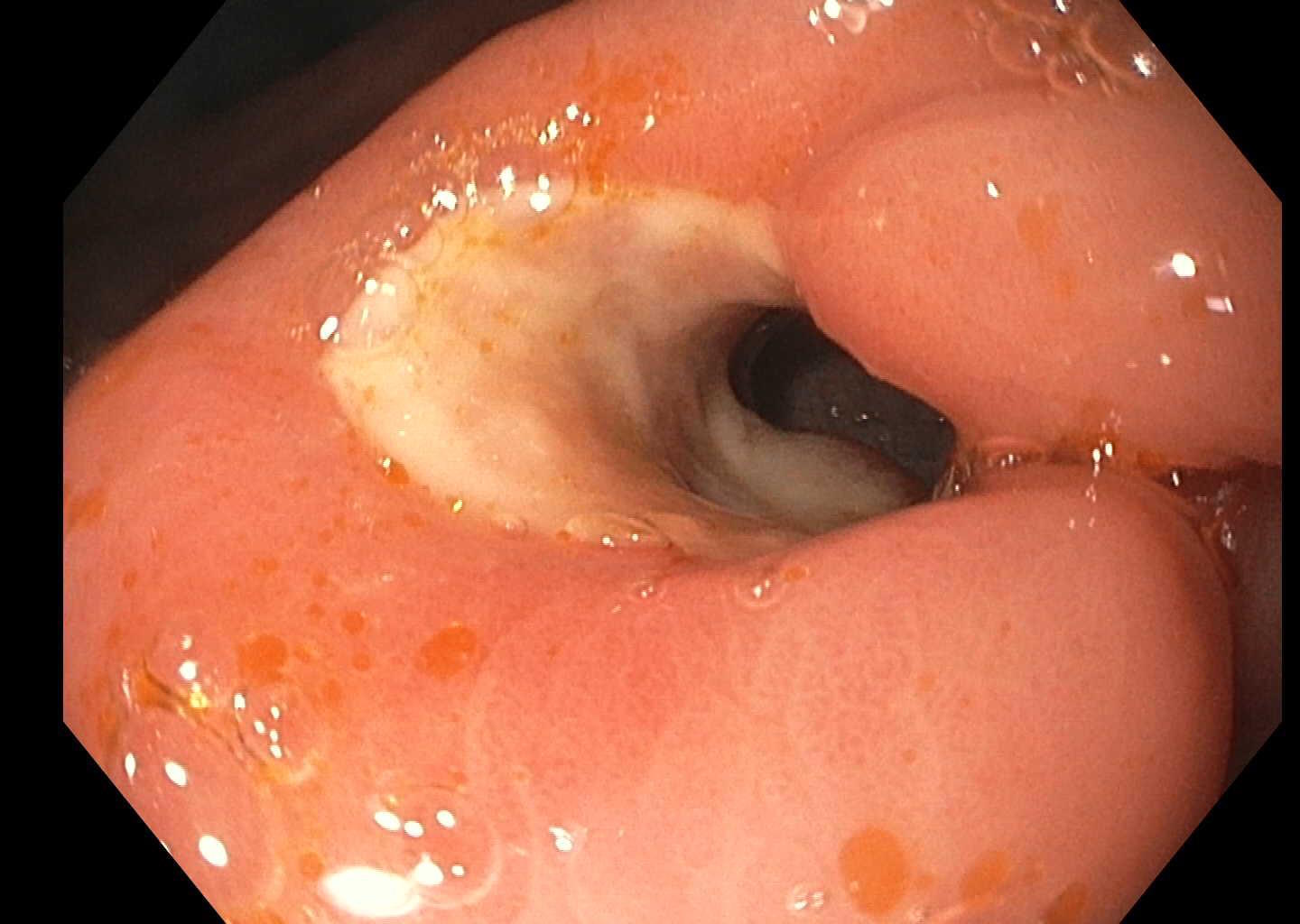
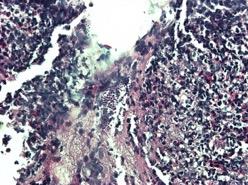
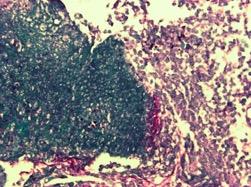
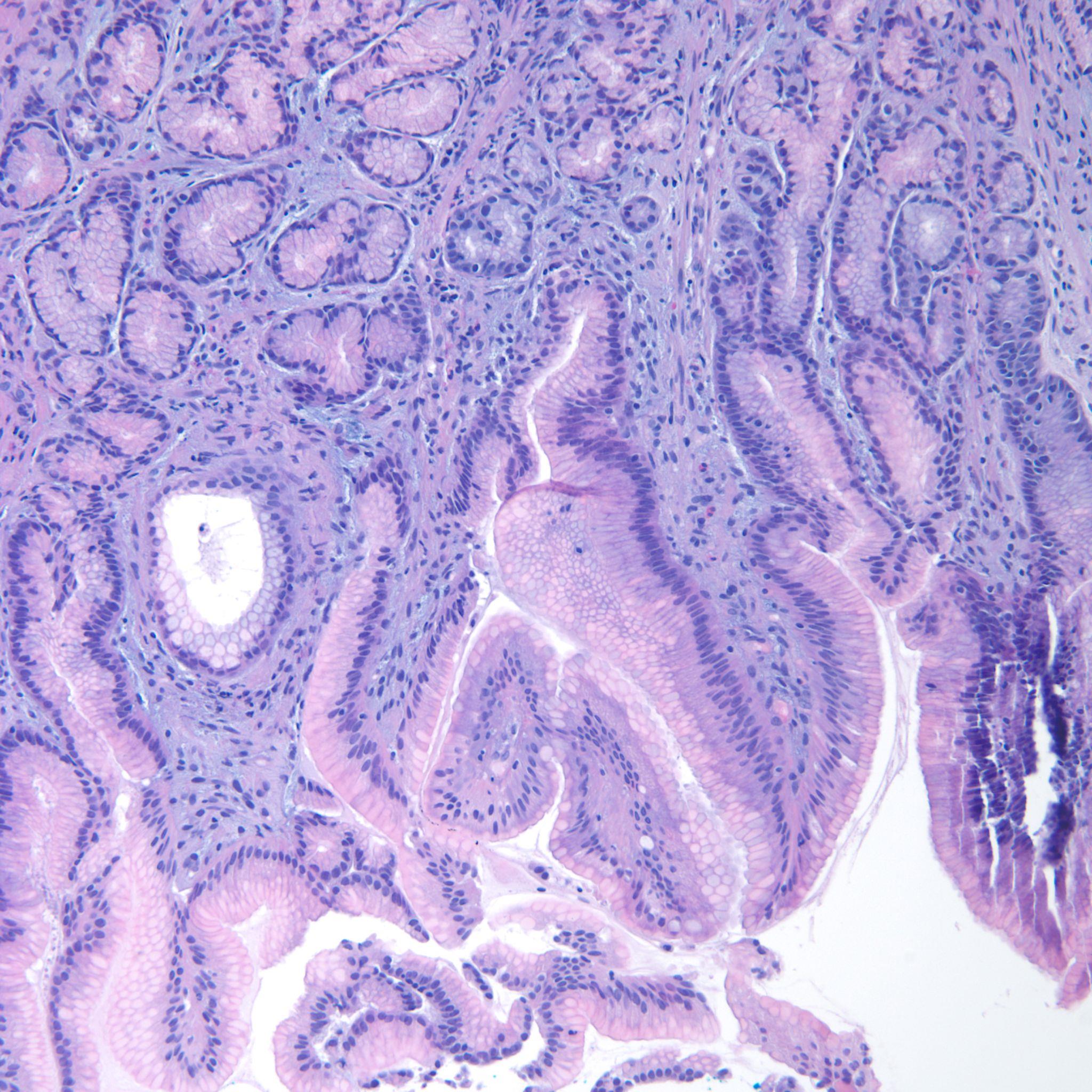
Esophagogastroduodenoscopy (EGD) revealed a 1 cm, non-bleeding, irregular-shaped, deep and clean-based ulcer at the pylorus (Fig. 1). The remainder of the examination was unremarkable. Biopsies were taken and revealed an ulcer with necro-inflammatory debris and fungal organisms, consistent with Candida species (Fig. 2). Periodic acid-Schiff (PAS) fungal stain revealed scattered yeast colonizing the fibrinous debris (Fig. 3).
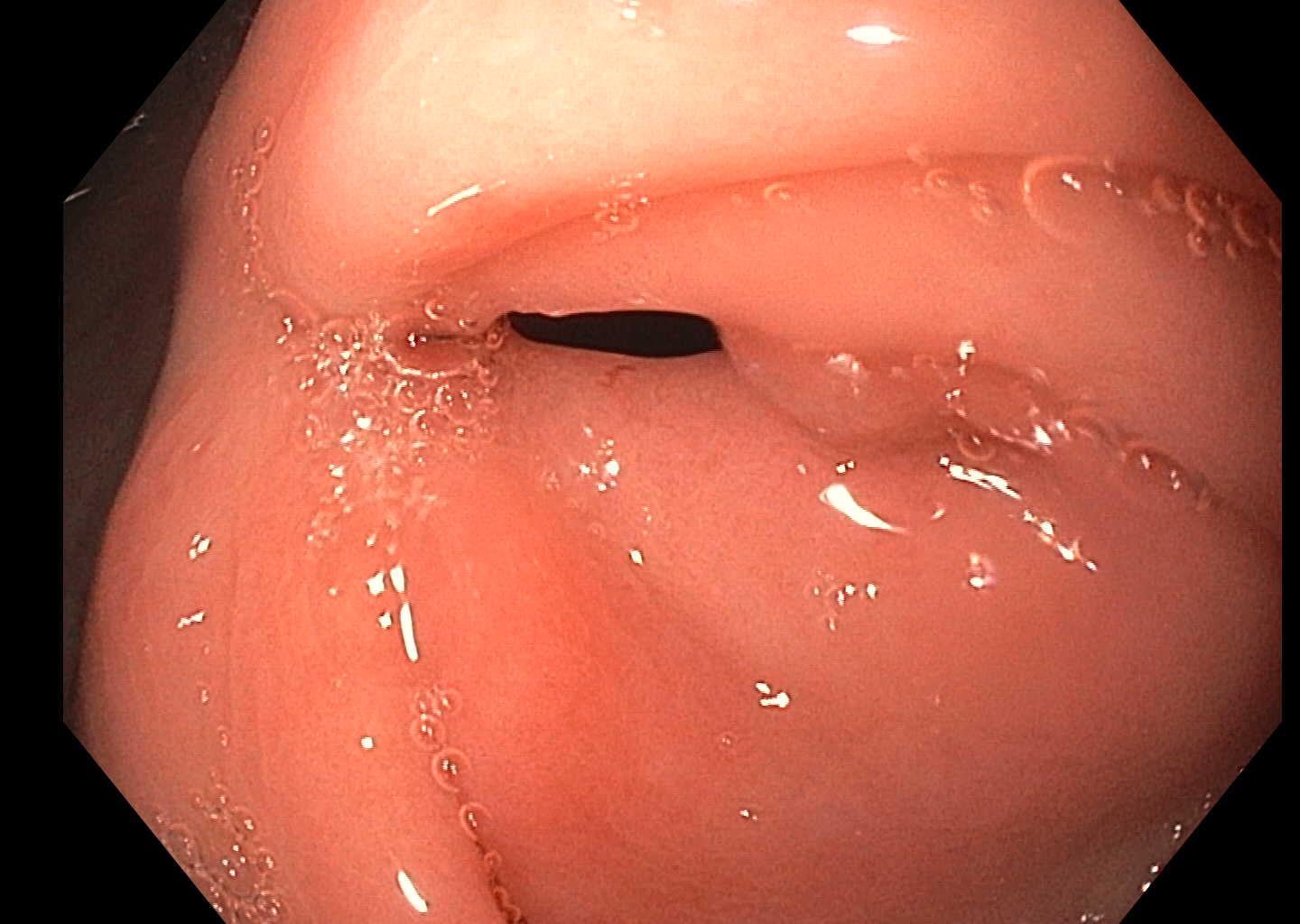
The patient was given a 3-week course of fluconazole and her symptoms had resolved on follow-up. Repeat EGD (2 months later) revealed resolution of her ulcer (Fig. 4. Repeat biopsies of the pylorus were negative for any evidence of fungal organisms (Fig. 5).
Figure 1. Initial endoscopy findings
Figure 2. H&E staining of biopsy specimen
Figure 3. Periodic acid–Schiff (PAS) staining of biopsy specimen
Figure 4. Endoscopy after treatment
Figure 5. Repeat biopsy after treatment
DISCUSSION
Peptic ulcers are breaks in the gastric or duodenal mucosa which penetrate through the muscularis mucosa and create a cavity with surrounding inflammation. Peptic ulcer disease (PUD) is the most common cause of stomach and duodenal perforation. Worldwide, there were 87.4 million new cases of peptic ulcers in 2015 resulting in 267,500 deaths [1]. PUD affects more than 6 million people in the USA each year [2].
A large, retrospective study using the National Inpatient Sample consisting of US inpatient data between 1998 and 2005 showed an average annual PUD hospitalization rate of 63.6/100,000 population.
Helicobacter pylori infection and NSAID use are responsible for the overwhelming majority of PUD cases. However, improved detection with endoscopy has reduced H. pylori prevalence. Other causes of non-H. pylori non-NSAID ulcers include antiplatelet drugs, stress, Helicobacter heilmannii, cytomegalovirus, Behçet’s disease, Zollinger-Ellison syndrome, Crohn’s disease and cirrhosis with portal hypertension [3]. Risk factors for the development of PUD are the use of NSAIDs, H. pylori, COPD, chronic renal insufficiency and tobacco use. Even though fungal PUD has a prevalence of 4–36%, the diagnosis is frequently overlooked [4].
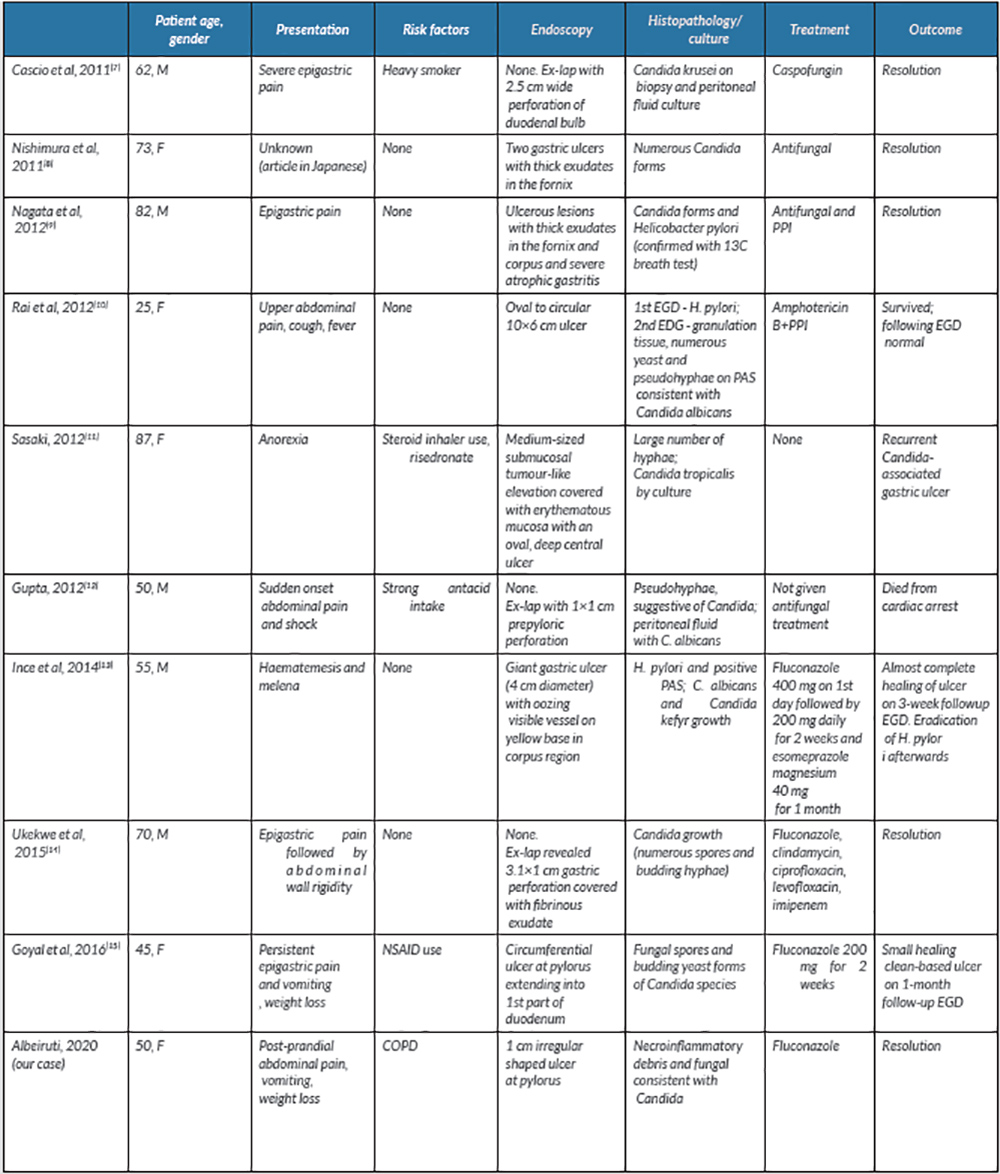
Candida is a normal commensal organism in the gut and colonizes the oesophagus in 20% of healthy adults [5]. Few cases of fungal PUD in immunocompetent patients have been reported over the past 10 years (Table 1). A review of 16 patients between 1998 and 2007 at a university hospital in Korea revealed that nine cases of gastric candidiasis were benign ulcers and the other seven were malignant. Similar to previous literature, associated conditions included diabetes, cirrhosis, lung cancer and pulmonary tuberculosis [6].
A literature review yielded 10 cases of fungal PUD in immunocompetent patients with risk factors such as smoking, steroid use and heavy antacid use. Antifungal treatment resulted in clinical improvement and ulcer resolution in eight of the 10 patients. One patient, with a perforated fungal ulcer, died post-operatively after cardiac arrest and did not receive any medication. Another patient refused treatment and interestingly was found to have a recurrent Candida-associated gastric ulcer in a different location. One patient was found to have H. pylori on an initial biopsy of a peptic ulcer, followed by Candida albicans on the second endoscopy. Two patients had co-existing infection with both H. pylori and C. albicans and were both successfully treated with an antifungal agent and a proton-pump inhibitor. Patients with large ulcers may have fungal PUD. Overall, the treatments were varied as regards lengths of treatment and antifungal agents which included fluconazole, caspofungin and amphotericin B (Table 1). Our patient eventually achieved clinical and biopsy-proven resolution after completing a course of fluconazole, providing more evidence for the use of antifungals in the treatment of Candida PUD.
Table 1. Published cases of fungal peptic ulcer disease
COPD, chronic obstructive pulmonary disease; EGD, esophagogastroduodenoscopy; ex-lap, exploratory laparotomy; F, female; M, male; NSAID, non-steroidal anti-inflammatory drug; PAS, periodic acid–Schiff; PPI, proton-pump inhibitor.
CONCLUSIONS
We present a case of EGD and biopsy-proven resolution of PUD secondary to Candida infection in an immunocompetent patient. It is important for clinicians to maintain a higher index of suspicion for other causes of PUD for correct and prompt management.