ABSTRACT
Bacille Calmette–Guérin (BCG) administration for superficial bladder cancer is a well-tolerated and very effective therapy. However, unpredictable systemic complications may occur on rare occasions. We present the case of a patient who attended for consultation because of fever, asthenia and weight loss following BCG immunotherapy. The clinical response to treatment and computed tomography scanning were key to diagnosis.
LEARNING POINTS
- It is essential to keep a high index of suspicion of possible, although uncommon, complications in patients treated with BCG immunotherapy.
- Response to treatment should always be evaluated to confirm diagnostic suspicion.
KEYWORDS
BCG infection, miliary tuberculosis, hepatitis
CASE DESCRIPTION
A 73-year-old man was referred for an Internal Medicine consultation because of a 2-week history of fever, weight loss and asthenia. He had been diagnosed 2 years previously with a superficial bladder tumour and transurethral resection had been performed. For the last year he had been on maintenance treatment with 3-weekly instillation of BCG every 3 months for the last 10 months. Recent cystoscopy had revealed no evidence of tumour recurrence.
When the symptoms started, the patient had attended the emergency room for fever and confusion. At that time, he was diagnosed with a urinary tract infection, so third generation cephalosporin treatment was initiated and he was discharged home. However, fever persisted as well as anorexia and weight loss and he was therefore referred for an Internal Medicine consultation. The patient also mentioned a discrete dry cough for the last couple of days. Physical examination only revealed a decrease in vesicular breath sounds. Blood tests showed elevation of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and a rise in liver enzymes. The remainder of the blood parameters were normal (including blood count, thyroid hormones, proteins and autoimmune study). Urinary sediment analysis showed minimal leukocyturia with urine culture persistently negative. Serological test results for hepatotropic viruses were not compatible with acute infection.
Chest x-ray was normal and abdominal ultrasound showed diffuse homogeneous hepatomegaly. The patient was admitted to hospital for further study.
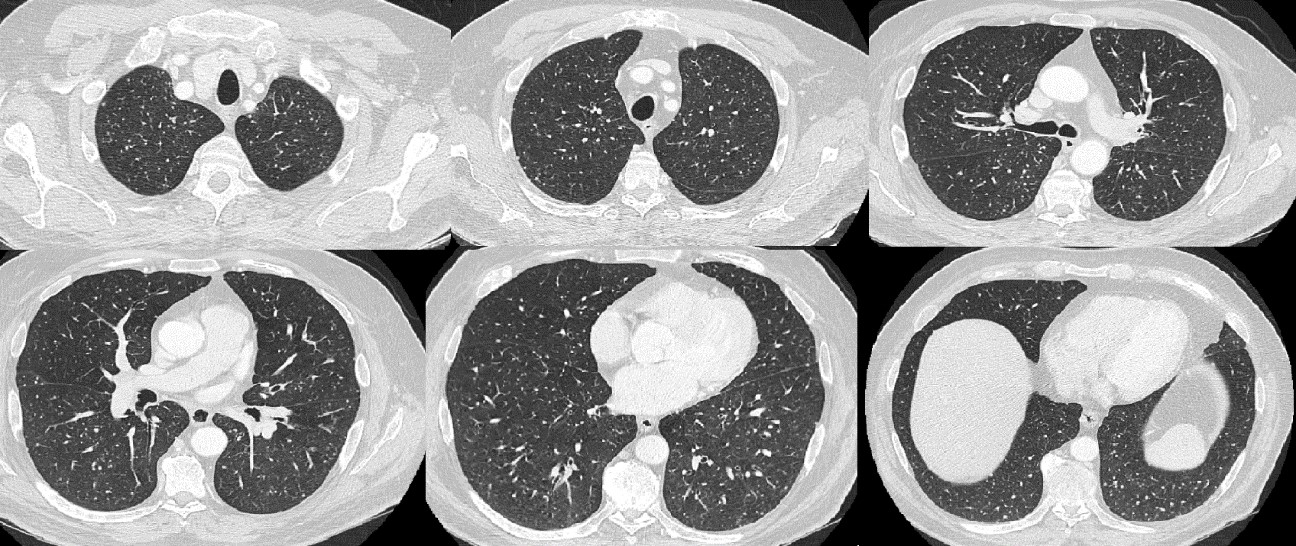
A computed tomography (CT) scan (Fig. 1) was performed and showed numerous, small pulmonary nodules scattered throughout the lungs, consistent with miliary tuberculosis. Polymerase chain reaction for Mycobacterium tuberculosis complex was negative. In line with the clinical findings and CT scan results, treatment with 300 mg isoniazid, 600 mg rifampin and 1200 mg ethambutol orally once a day was started. Mycobacterium bovis, BCG strain, was then identified by liquid culture medium in several urine samples, weeks after the last BCG instillation. The patient was discharged home once his general condition had improved and fever had resolved.
Figure 1. CT scan showing small pulmonary nodules scattered throughout the lungs.
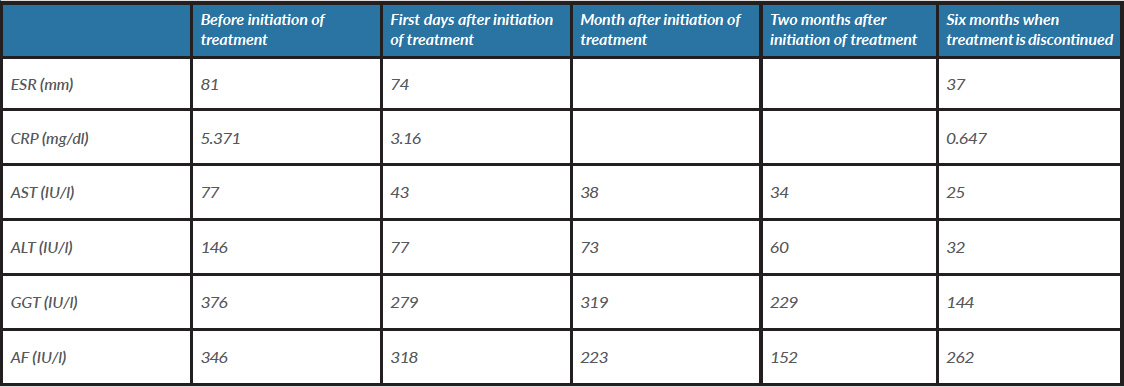
During follow-up visits, the patient referred progressive resolution of anorexia and asthenia, and periodic blood tests demonstrated normalization of liver enzymes and acute phase reactants (blood test results are shown in Table 1). Ethambutol was discontinued after 2 months of treatment and isoniazid and rifampicin after 6 months. Although it was not possible to identify the BCG strain in sputum culture, the definitive diagnosis was disseminated BCG infection, defined by miliary tuberculosis and liver involvement.
Table 1. Evolution of test results during treatment
AF, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GGT, gamma-glutamyltransferase.
DISCUSSION
We present a case of miliary tuberculosis and hepatitis as rare complications following intravesical BCG administration. BCG therapy consists of intravesical instillation of viable attenuated mycobacteria and is used in bladder cancer treatment. This procedure is considered a very successful immunotherapy, and works by inducing a robust cellular immune response that attack tumour cells. This therapy eradicates the existing tumour and also reduces frequency of tumour recurrence [1, 2].
Systemic complications are unusual, with an approximate incidence of 4%. Clinical manifestations may range from miliary tuberculosis to hepatitis, nephritis or sepsis. It is not clear if the mechanism underlying these complications is a form of hypersensitivity reaction or a direct result of mycobacterial infection. One hypothesis suggests that haematogenous dissemination of the bacilli through the disrupted urothelial barrier may play a role in the development of this entity [3, 4].
Diagnosis is not easy, and commonly missed in the initial differential diagnosis. In a pooled analysis of cases of BCG infection, the diagnosis was supported by microbiological procedures in less than 50% of cases, while histological findings were present in 65% of cases [3]. Microbiological tests have low sensitivity, and more conclusive results may be achieved by taking a biopsy sample, resulting in more invasive diagnostic tests.
M. bovisidentification in urine culture has a low positive predictive value. In asymptomatic patients, it is possible to find persistence of BCG in the bladder by mycobacterial culture and/or PCR assays, weeks after instillation. The chances of positive results are lower the longer time since the procedure [5].
CT scanning played an important role in diagnosis in our patient, while the chest x-ray was reported as normal. A CT scan should be preferred over conventional chest radiography as in 25% of previous case reports chest radiography failed to reveal a miliary pattern [3].
Nevertheless, the evident response to antituberculosis drugs confirmed the diagnosis in this case. According to the literature, diagnosis was based only on clinical manifestations and response to antituberculosis treatment in 17 previous case reports [3].
As in other cases of disseminated BCG infection, three anti-tuberculosis drugs were employed. BCG is generally highly sensitive to isoniazid, rifampin, ethambutol and intrinsically resistant to pyrazinamide [3, 6]. Possibly related to a hypersensitivity reaction, there have been similar cases of disseminated BCG infection when corticosteroids were added to antituberculosis treatment in order to reduce fever and induce clinical improvement [3, 4].
Although disseminated BCG infection is a potentially life-threatening complication of a potentially life-saving therapy, it is also uncommon. Professionals should be aware of the development of symptoms and suspect this entity in patients submitted to BCG therapy.