ABSTRACT
Inflammatory myofibroblastic tumour (IMT) is a rare mesenchymal tumour. It is usually benign but may behave as a malignant tumour with multiple recurrences and metastases. We present the case of a young woman with weight loss associated with diffuse abdominal pain, who was shown to have a large pancreatic mass. Investigation revealed fusocellular mesenchymal neoplasia, compatible with the diagnosis of IMT. As the mass was unresectable, glucocorticoid therapy was initiated with an excellent response and regression of the tumour.
LEARNING POINTS
- Inflammatory myofibroblastic tumour (IMT) is a rare entity.
- Since IMT can occur in different locations, its clinical presentation is varied, with non-specific symptoms.
- Prognosis is usually benign, but relapse and metastasis have been reported.
KEYWORDS
Abdominal pain, retroperitoneal space, neoplasm, inflammatory myofibroblastic tumour
INTRODUCTION
The aetiology of inflammatory myofibroblastic tumour (IMT) has not yet been fully clarified [1]. It was described for the first time in the lung by Brunn in 1934 but only recognised recently as a distinct pathological entity among the group of inflammatory pseudotumours [2, 3]. It has a higher prevalence in children and young adults and preferentially affects the lung and abdominal cavity, with a retroperitoneal location being rare [1, 4, 5].
CASE DESCRIPTION
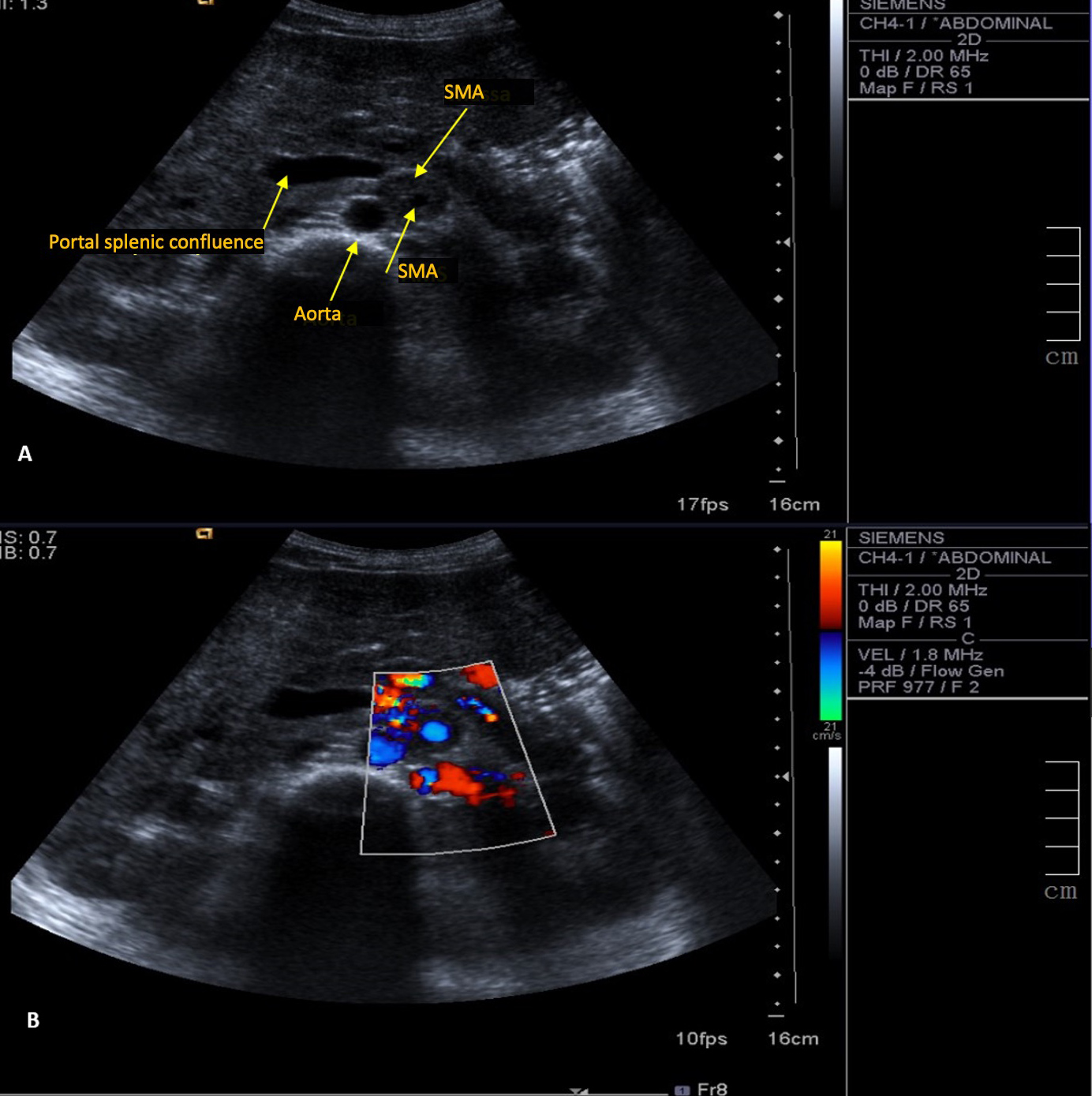
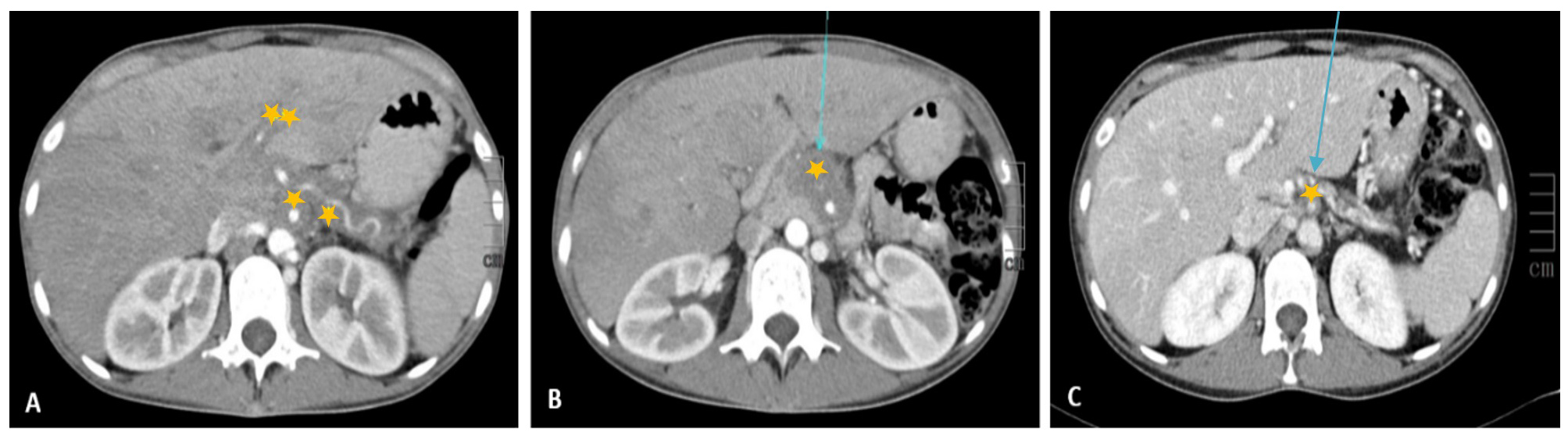
A 30-year-old woman with leukoderma was referred to the emergency department because of weight loss of over 10% (6 kg) of her body weight in 6 months, associated with diffuse abdominal pain. Physical examination revealed an anicteric and acyanotic patient with weight loss and bleached but hydrated mucous membranes. Abdominal palpation caused diffuse discomfort but no peritoneal irritation and no palpable masses or organomegaly were noted. Blood analysis demonstrated normochromic normocytic anaemia (10 g/dl). Liver tests showed a cytocholestase pattern with aspartate transaminase (AST) 125 U/l, alanine transaminase (ALT) 215 U/l, alkaline phosphatase 1543 U/l, gamma-glutamyltransferase (GGT) 984 U/l, and total/direct bilirubin 1.09/0.77 mg/dl, without increased inflammatory parameters. An abdominal ultrasound showed a large mass wrapped completely around the superior mesenteric artery, without occlusion (Fig. 1). An abdominal CT scan revealed a large pancreatic mass extending from the body to the tail of the pancreas, with invasion of the celiac trunk, splenic hepatic artery and splenic confluence, suggestive of lymphoproliferative disease (Fig. 2).
Figure 1. Ultrasound (A) and colour Doppler (B) images. A mass is wrapped completely the superior mesenteric artery (SMA) without causing obstruction
Figure 2. Contrast-enhanced CT scan of the abdomen and pelvis in the arterial phase (A and B) and portal phase (C). A hypodense mass (*), with soft- tissue densities, in the pancreatic/peripancreatic region is completely wrapped around the superior mesenteric artery and the splenic artery, without causing compression. The mass displaces the structures of the median line to the right. Hepatomegaly was found with the periportal halo sign (**), likely indicating obstruction of lymphatic drainage
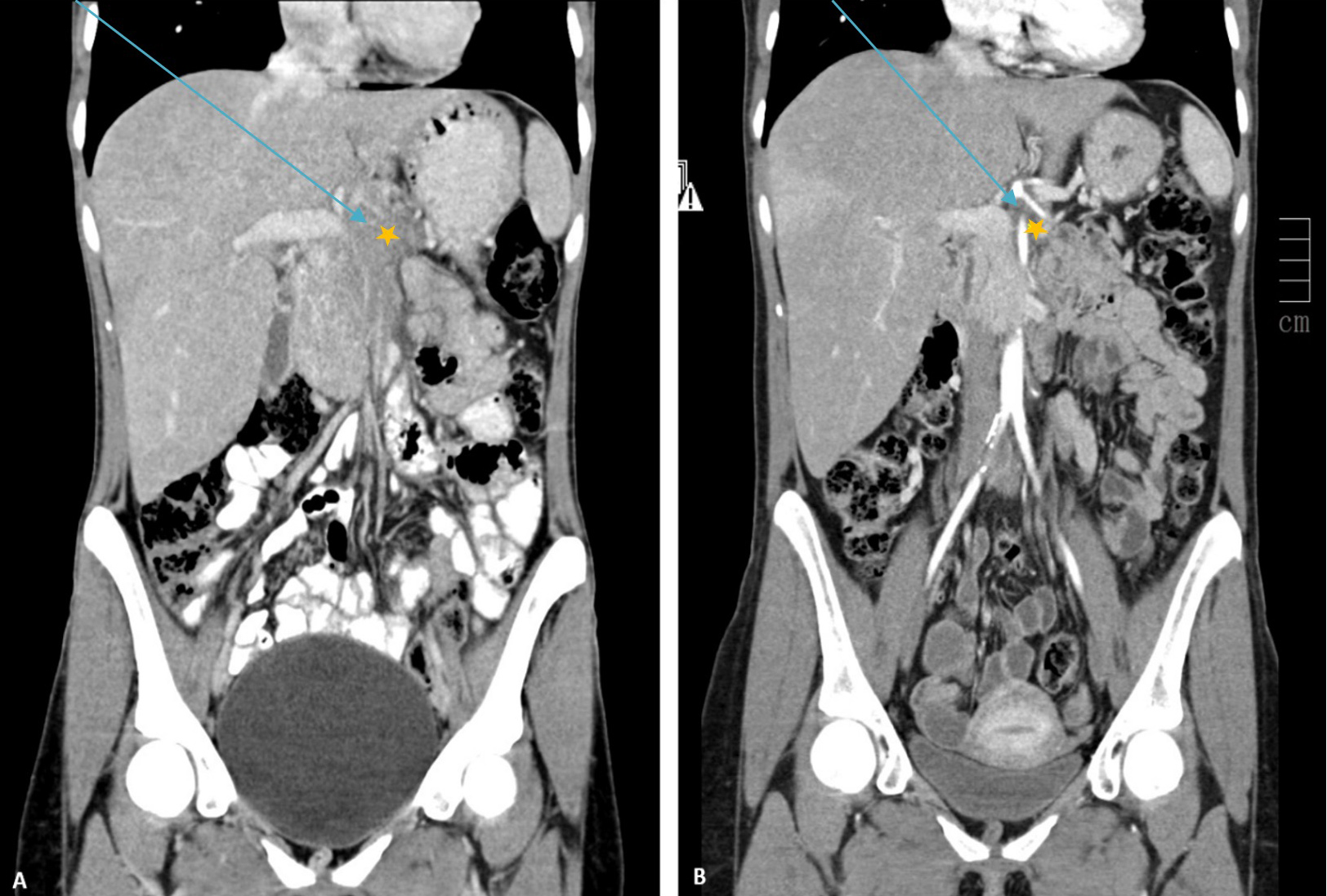
Subsequent echo-endoscopy was performed and a biopsy of the lesion was taken. Cytology revealed non-specific fibrotic and inflammatory tissue. Upper gastrointestinal endoscopy revealed gastric varices in the fundus of the stomach, without further changes. Tumour markers were negative (CEA, CA 19.9 and B2-microglobulin). The patient refused hospital admission, but later attended the emergency department several times because of worsening abdominal pain, with subsequent need for transdermal opioids. The patient continued to lose weight, dropping to 39.5 kg (from an initial weight of 50 kg) and developed jaundice. Examination of a second biopsy revealed low-grade fusocellular mesenchymal neoplasia of intermediate malignant potential, with pancreatic parenchymal infiltration without destruction of glandular structures, compatible with the diagnosis of IMT. Tests revealed no ALK gene rearrangement. As the tumour was unresectable, glucocorticoid therapy was initiated (prednisolone 20 mg per day) with a favourable response. Eleven months after starting treatment, the patient was pain-free, anicteric, and weighed 59.9 kg, but had facial plethora. Radiological follow-up showed a residual 5 mm lesion around the superior mesenteric artery (Fig. 3). The glucocorticoid dose was gradually reduced to 5 mg/day without associated toxicity.
Figure 3. Contrast-enhanced CT image of the abdomen and pelvis in the arterial phase (coronal plane) before (A) and after (B) glucocorticoid therapy. A significant reduction in the size of the hypodense mass (*) was seen, with less deviation of the structures of the median line
DISCUSSION
IMT is a normally benign tumour. Recurrence rates range between 2% and 25% and metastases have been reported in 5% of patients [4]. Although IMT has a predilection for the lung, it can present in many other locations, such as the retroperitoneal space, bladder, kidney, spleen, breast, colon, prostate, peripheral nerves and soft tissues [6]. It therefore has a varied clinical presentation, with vague and non-specific symptoms [7]. When it is located in the abdomen, the most frequent symptoms are abdominal pain, fever, weight loss, anorexia, dysphagia, constipation or even intestinal occlusion. However, IMT can be asymptomatic in 15–40% of patients [8]. In about one third of IMT patients, anaemia, thrombocytopenia, polyclonal hyperglobulinemia and an increased erythrocyte sedimentation rate may be observed [2]. Radiologically there is no pathognomonic finding for this type of tumour, but there may be an ill-defined image of an infiltrative lesion or a lesion within well-defined limits [9].
The definitive diagnosis of IMT is obtained through biopsy. Three histological types are described: myxoid/vascular, fusocellular (as observed in this case) and fibrous hypocellular. Infiltration of plasma cells, lymphoid aggregates and eosinophils are observed in all three types [10]. Alterations involving the ALK gene (anaplastic lymphoma kinase), typically associated with anaplastic large cell lymphoma, have been observed in 50% of patients with IMT [4]. The definitive treatment for IMT is surgical resection where possible. Glucocorticoid therapy is administered to patients with unresectable tumours or when complete removal is not possible, with chemotherapy being reserved for patients with invasive or recurrent tumours [11]. The prognosis is usually benign, but cases of relapse and metastasis have been reported, and IMT may behave like a malignant entity [8]