ABSTRACT
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease and has emerged as a serious public health challenge. About 20% of NAFLD patients may have low titres (<1:320) of antinuclear antibodies (ANA). However, we describe a patient with NAFLD whose ANA titre was high (>1:320) on presentation. After 3 months of diet, exercise and vitamin E supplementation,the patient was symptomatically better but her ANA titre had increased (>1:640). Her liver biopsy showed features of NAFLD with minimal fibrosis. High-titre ANA (>1:320) positivity is rare. Our patient showed a progressive rise in ANA titre from >1:320 to >1:640 within 3 months even though she was improving and histology showed minimal fibrosis.
LEARNING POINTS
- Up to 30% of patients with non-alcoholic fatty liver disease (NAFLD) may have antinuclear antibodies (ANA).
- Low titre (<1:320) ANA positivity is not uncommon, but a high titre is rare.
- The ANA titre does not correlate with the histological grade of NAFLD.
KEYWORDS
Antinuclear antibody, non-alcoholic fatty liver disease, autoimmune hepatitis, liver biopsy
CASE DESCRIPTION
A 49-year-old woman presented with gradual onset of anorexia, nausea, upper abdominal fullness and fatigue for the previous 2 months. She had no history of fever, night sweats, weight loss, joint pain, rash, dry eye or swelling of the face. Bowel and bladder habits were normal. There was no history of black stool or fresh blood in the stool. The patient did not smoke or drink alcohol. Her menstrual history revealed no abnormality. She had a 1-year history of hypertension which was treated with telmisartan 40 mg once daily. She had no history of diabetes or dyslipidaemia. However, she did have a family history of cirrhosis of the liver but did not know the aetiology of her elder brother’s disease.
On examination, there was no jaundice. Her vital signs (e.g., pulse, blood pressure and respiratory rate) were all normal. She weighed 68 kg and her BMI was 28.2 kg/m2. There was no generalized lymphadenopathy. Examination of the breast was normal. Abdominal examination revealed 2 cm firm, non-tender, smooth-surfaced hepatomegaly without any splenomegaly. There were no stigmata of chronic liver disease. The rest of the systemic examination was normal.
On investigation, a complete blood count, C-reactive protein, urea, creatinine and coagulation profile were normal. A liver function test (LFT) revealed total bilirubin 1.3 mg/dl (direct 0.3 mg/dl, indirect 1 mg/dl), ALT 100 U/l, AST51 U/l, serum albumin 4.5 g/dl and globulin 3.4 g/dl. Serum alkaline phosphatase and GGT were normal. Ultrasonography of the upper abdomen showed a bright echogenic liver consistent with fatty liver disease (grade I–II). A hepatic FibroScan revealed a median value of 5.6 kPa, which indicates no fibrosis. A provisional diagnosis of NAFLD was made. The patient was advised to follow a strict fat-restricted diet, exercise and take vitamin E 400 mg once daily.
As the patient was female and had a family history of liver cirrhosis, we wished to rule out autoimmune hepatitis (AIH) and so ordered Liver Profile including auto immune markers. Surprisingly, ANA-HEp-2 was positive (>1:320 titre and centromere pattern) by the indirect immunofluorescence method. Serum ferritin, alpha-1-antitrypsin, transferrin saturation, alpha fetoprotein, anti-mitochondrial antibody, anti-LKM antibody and serum ceruloplasmin all were negative. To rule out other collagen vascular diseases, blood was sent for ANA profiling but all findings, including anti-dsDNA, were negative. The patient did not give consent for a liver biopsy. She was advised to attend a follow-up consultation 3 months later.
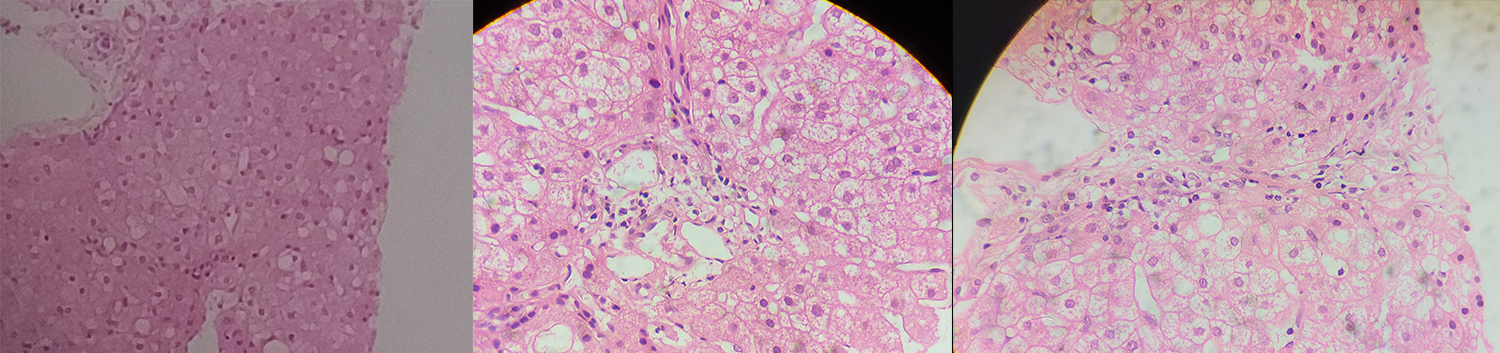
After 3 months, the patient was symptomatically better. She still had 1 cm hepatomegaly and had lost 5 kg in weight. Repeat LFT showed improvement in ALT (decreased from 100 U/l to 74 U/l), but AST had marginally increased from 51 U/l to 53 U/l, while other LFT results were normal. Repeat blood tests for ANA-HEp-2 showed persistent positivity with a high titre of >1:640. After counselling, patient gave consent for a liver biopsy. The histology report was consistent with NAFLD (steatosis score 1, lobular inflammation score 1, ballooning score 0, fibrosis score 2, NAFLD activity score 2) (Fig. 1).
Figure 1. Microscopic view of liver tissue (hematoxylin-eosin, x400) showing fat infiltration of 30-40% of hepatocytes, minimal lobular inflammation and no ballooning cells (NAFLD activity score 2) consistent with steatosis and interface hepatitis. Minimal pericellular and periportal fibrosis (Fibrosis score 2)
Courtesy to Dr Sourav Bhowmik, Department of Pathology, Ruby General Hospital, Kolkata, India
DISCUSSION
Patients with suspected rheumatic disease are screened for ANA, which are sometimes also found in non-rheumatic conditions [1] including (a) liver disease (chronic hepatitis, primary biliary cirrhosis, alcoholic hepatitis, NAFLD/NASH), (b) infections (tuberculosis, viral hepatitis, syphilis, parasitic disease), (c) autoimmune disease (type 1 diabetes, Addison’s disease, autoimmune anaemia, Hashimoto’s disease), (d) skin disease (psoriasis, lichen planus),and (e) cancer (breast cancer, prostate cancer, leukaemia, Hodgkin’s lymphoma).
Although low ANA titres are seen in up to a third of patients with NAFLD/NASH, titres above 1:320 are rare. Therefore, ANA positivity does not always exclude NAFLD/NASH. In patients with suspected NAFLD, if ANA or anti-smooth muscle antibodies (ASMA) titres are above 1:160 and 1:40, respectively, a liver biopsy should be considered to exclude autoimmune disease [2, 3].
There are very few studies on the prevalence, influence and outcome of ANA positivity in NAFLD/NASH. In a cross-sectional study by Adams et al., the prevalence of ANA was 20% among NAFLD patients. Positive autoantibodies were associated with higher fibrosis stage, higher inflammatory grade and higher levels of gammaglobulin. The authors concluded that routinely measured autoantibodies are present in one quarter of patients with NAFLD and are associated with more severe histological damage. A liver biopsy is required to rule out AIH in most NAFLD patients with positive autoantibodies [4].
Ravi et al. conducted a study on the influence of autoimmune (AI) markers on the clinical presentation, natural history and outcome of steatohepatitis-related liver disease [5]. Of 607 patients (401 NAFLD),AI markers were available in 398 (mean age 50±15 years, 52% male, median body mass index (BMI) 38, 44% with diabetes, 62% with non-alcoholic steatohepatitis (NASH), median MELD score 9). A total of 78 (19.6%) patients were positive for AI markers without differences between alcoholic liver disease (ALD)and NAFLD, cirrhosis and no cirrhosis, and NASH and no NASH. There were no differences regarding age, gender, BMI, cirrhosis at presentation, MELD score, endoscopic findings or histology based on AI markers. However, serum ALT was higher among patients with AI markers (65±46 vs.59±66 IU/l; p=0.048). The data remained unchanged when patients with NAFLD were analysed. None of the 11 ANA-positive patients (four with a titre >1:640) had findings indicating AIH. On median follow-up of about 3 years, there were no differences in liver disease outcomes (ascites, encephalopathy, variceal bleeding), hepatocellular carcinoma, transplantation or survival.
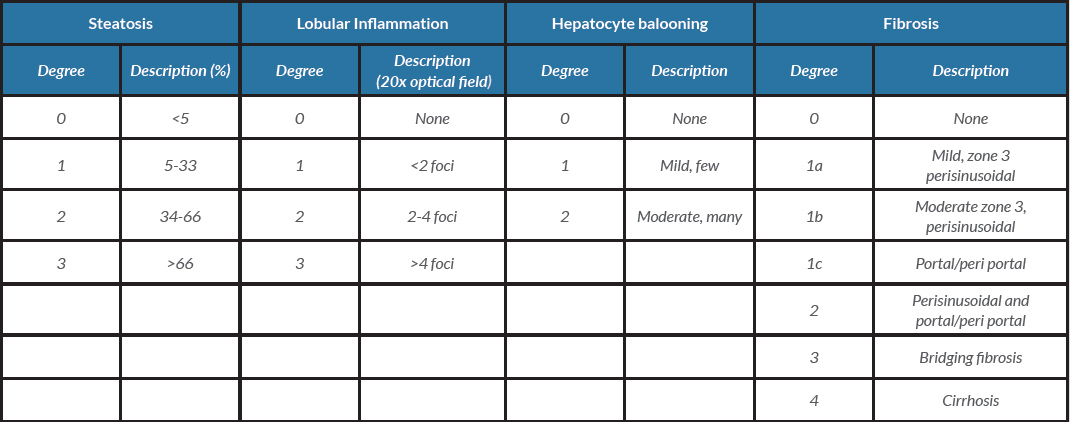
Liver biopsy is the gold standard for both diagnosis and disease staging. NASH is defined by the presence on histology of hepatocellular injury (ballooning, apoptosis/necrosis, Mallory’s hyaline and giant mitochondria) and inflammation (neutrophils and other inflammatory cell infiltrate). The most widely used scoring system is the NASH Clinical Research Network (CRN) activity score (NAS), which grades steatosis, lobular inflammation and hepatocellular ballooning, to rate liver fibrosis on a score of 0–4 [6, 7] (Table 1).
As our patient was a woman with a family history of liver cirrhosis of unknown aetiology, histological confirmation of liver disease was required. An extensive search of the literature did not reveal any other reports of a progressive high ANA titre in a patient with NAFLD. We have described a rare case of NAFLD with high-titre ANA positivity but also with clinical improvement and low-grade fibrosis according to the NASH CRN scoring system [7].
Table 1. Nonalcoholic Steatohepatitis Clinical Research Network scoring system
Courtesy to Dr Sourav Bhowmik, Department of Pathology, Ruby General Hospital, Kolkata, India
CONCLUSION
The presence of ANA in NAFLD patients is generally thought to be an epiphenomenon and studies examining the relationship between disease severity and the presence of autoantibody have reported conflicting results. Progressive high-titre ANA positivity (>1:320 to >1:640) can be present in NAFLD. It is prudent to exclude AIH by liver biopsy and histology, as management is different. A high titre of ANA does not necessarily indicate severe NAFLD.