ABSTRACT
Patients affected by COVID-19 pneumonia may develop stress cardiomyopathy, also known as Takotsubo syndrome (TTS), at different stages during the disease and with different degrees of left ventricular dysfunction. We describe three cases of TTS in COVID-19-positive patients with different clinical presentations and outcomes. One of them died, while in the other two coronary angiography confirmed the diagnosis but was postponed until after pneumonia resolution because of the risk of virus spread.
LEARNING POINTS
- An association between COVID-19 and cardiac involvement is highlighted.
- The incidence of Takotsubo syndrome has increased during this pandemic, possibly because it is caused by acute stress.
KEYWORDS
COVID-19, Takotsubo syndrome, cardiomyopathy
INTRODUCTION
Takotsubo syndrome (TTS) is a stress cardiomyopathy induced by intense emotional or physical stress leading to rapid, severe, and usually reversible, cardiac dysfunction, and accounts for 1.8–2.2% of all acute coronary syndromes [1]. There are suggestions that TTS may be associated with COVID-19 [2].
CASE DESCRIPTION
We describe three cases of stress cardiomyopathy in patients recently admitted to the Covid hospital in Dolo, near Venice in Italy, for severe acute respiratory disease syndrome due to COVID-19 infection. They all had a history of appropriately treated arterial hypertension or diabetes, and were in good clinical condition before hospitalization according to the Clinical Frailty Scale [3] (Table 1). None of them had previous history of cardiovascular disease and had common symptoms of infection including fever, cough with dyspnoea and atypical chest pain during the 10 days before their attendance at the emergency department (ED).
All three nasopharyngeal swabs taken in ED were positive for COVID-19. Blood samples were analysed for high-sensitivity troponin T (hs-cTn), NT-proBNP and D-dimer after patients were admitted and also if their clinical condition worsened or the electrocardiogram (EKG) showed changes. All three patients were promptly treated with antiviral drugs and hydroxychloroquine and a daily EKG was performed to check QT interval duration. The pulmonary CT scan in all three patients was positive for ground-glass opacities and bilateral consolidation in the lungs. All patients had high D-dimer values or clinical features indicating thromboembolic acute events but none had symptoms, EKG findings, hs-cTn values or echocardiographic results suggesting ischaemia. Because of evidence of hypercoagulation in COVID-19 patients, subcutaneous fondaparinux 2.5 mg was administered together with other treatment mandated by hospital protocol.
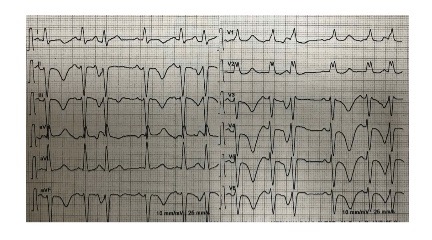
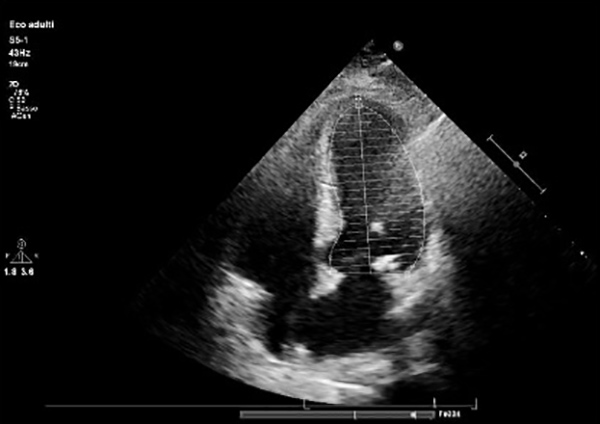
Fig. 1) and a typical echocardiographic pattern characterized by dyskinesia of the left ventricle apex (apical ballooning) and basal wall hypercontractility with systolic dysfunction (Figs. 2 and 3).
Table 1. Clinical characteristics
CRP, C-reactive protein; HF, high flow; Hs-cTn, high sensitivity troponin T; iv, intravenous; sc, subcutaneous.
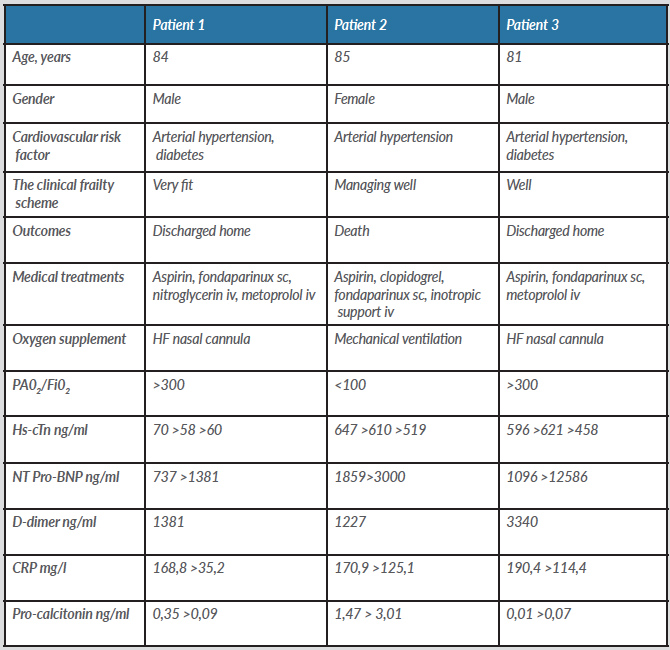
Figure 1. EKG of patient 1
Figure 2. End diastolic left ventricular volume
Figure 3. End systolic left ventricular volume with typical apical ballooning
The first patient was an 84-year-old man with a normal PaO2/FiO2 ratio, who was treated with high-flow oxygen therapy via a nasal cannula. During hospitalization, he had sudden worsening of dyspnoea and chest pain caused by a hypertensive crisis with a blood pressure (BP) reading of 220/100 mmHg. Nitroglycerin and intravenous metoprolol were promptly administered with progressive improvement of symptoms and normalization of BP. hs-cTn was high at 70 ng/ml but did not show a typical ischaemic increase. The EKG and ultrasound showed typical TTS with a global preserved left ventricular ejection fraction (EF) of 53%. Because of significant COVID-19 lung involvement, coronary angiography was postponed for a couple of weeks until pneumonia had completely resolved, to avoid possible cardiovascular complications and virus spread. Aspirin was started before coronary angiography, which was negative for significant coronary stenosis.
The second patient was an 85-year-old woman who was admitted to the Covid department with fever and cough. She presented septic shock due to Pseudomonas aeruginosa infection 2 days later and was transferred to the Intensive Care Unit. She had progressive respiratory failure (PaO2/FiO2 <100) which required mechanical ventilation, but blood oxygen saturation levels did not improve. We therefore performed an EKG and an echocardiogram which were highly suspicious for TTS. The echocardiography showed a severe reduction in LVEF (30%). Despite ventilation in the prone position, the use of plasma expander and inotropic support, the patient developed rapid multiorgan failure and died within a few hours. The autopsy confirmed a normal coronary anatomy.
The third patient was an 81-year-old man with a normal PaO2/FiO2 ratio who was supported with high-flow nasal cannula oxygen. Blood samples revealed high NT-proBNP levels and abnormal hs-cTn values but without an increase indicating ischaemia. EKG and echocardiography showed typical TTS alterations, with a moderately impaired LVEF (42%). Coronary angiography performed after pneumonia resolution was negative for significant coronary stenosis.
DISCUSSION
There are few reports of stress cardiomyopathy in the COVID-19 population. However, direct myocardial injury has been frequently described and attributed to myocarditis, hypoxaemia or underlying coronary artery disease. Coagulopathy and vascular endothelial dysfunction have also been highlighted as complications of COVID-19 infection [4].
Stress cardiomyopathy is usually triggered by extreme sympathetic stimulation with abnormal release of catecholamines and subsequent epicardial coronary spasm [5–7]. Various different mechanisms coexist in COVID-19 patients which may contribute to left ventricular dysfunction. This pandemic disease may induce stress manifesting as an increase in cases of stress cardiomyopathy. We would also like to emphasize that urgent coronary angiography in acute COVID-19 pneumonia could put the patient and the cardiologist at risk due to virus spread. In light of the possibility that COVID-19 patients may have acute coronary syndrome, we should maintain suspicion of stress cardiomyopathy and postpone invasive examination until after complete recovery from COVID-19 pneumonia.