ABSTRACT
Cytokine release syndrome (CRS) is a systemic inflammatory response that can be triggered by many factors such as infections. CRS in patients with coronavirus disease 2019 (COVID-19) is life-threatening and can occur very rapidly after COVID-19 diagnosis. Tocilizumab (TCZ), an interleukin‐6 (IL-6) inhibitor, may ameliorate the CRS associated with severe COVID‐19 and thus improve clinical outcomes. We present a case of life-threatening CRS caused by COVID-19 infection successfully treated with TCZ.
LEARNING POINTS
- Cytokine release syndrome (CRS) is a systemic inflammatory response that can be triggered by COVID-19.
- TCRS can be life-threatening in severe COVID-19.
- Tocilizumab may have a role in treating severe COVID-19 patients with CRS.
KEYWORDS
Tocilizumab, COVID-19, cytokine release syndrome
INTRODUCTION
SARS‐CoV‐2 causes a mild to moderate illness characterized by fever and respiratory symptoms, with or without evidence of pneumonia. However, up to 10% of patients with COVID‐19 may develop severe pneumonia with hypoxia, cytokine release syndrome (CRS) and multiorgan failure [1]. Tocilizumab (TCZ) is a humanized monoclonal inhibitor of the proinflammatory cytokine interleukin‐6 (IL‐6) and is licensed for use in the clinical management of CRS [2]. Peer‐reviewed data on the clinical use of TCZ in severe COVID‐19 are very limited. We describe a case of CRS caused by severe COVID-19 infection with a favourable response to TCZ therapy.
CASE DESCRIPTION
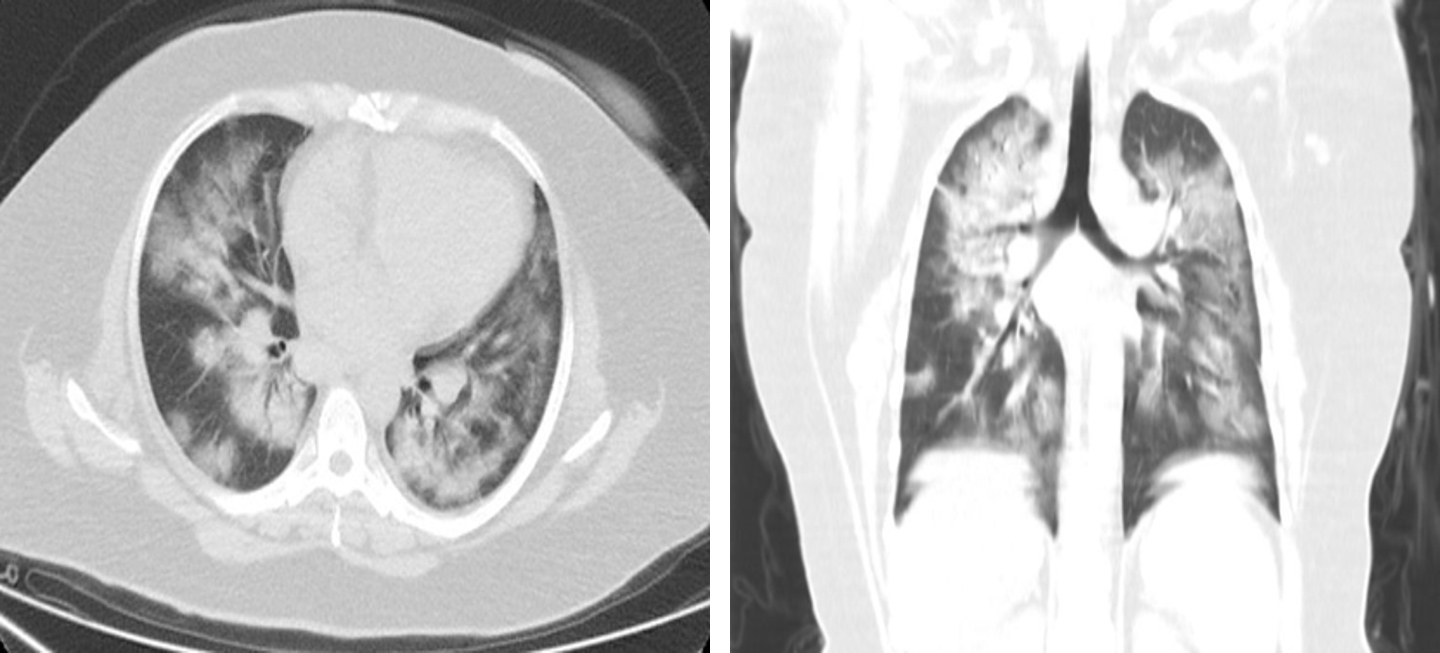
A 41-year-old woman with a history of hypertension, presented with fever, productive cough and general fatigue. She denied any recent travel but had a history of contact with sick people. In the emergency department she was tachypnoeic (respiratory rate of 22 breaths/min), tachycardiac (130 bpm), febrile (39°C) and hypoxic (oxygen saturation (SpO2) of 88% on room air). Physical examination of the lungs revealed bilateral crackles and wheeze. The white cell count was normal and the absolute lymphocyte count was low (0.61–103/µl), while the erythrocyte sedimentation rate (77; range, 0–20 mm/h) and C-reactive protein levels (110; range, 0–5 mg/l) were increased. D-dimer was 1657 ng/ml and procalcitonin was normal. Assays for influenza viruses and a respiratory syncytial virus were all negative. A nasopharyngeal swab was positive for SARS-CoV-2 on RT-PCR assay. Computed tomography (CT) at the time of admission revealed bilateral ground-glass opacities and consolidation (Fig. 1). The diagnosis of COVID-19 pneumonia was made and treatment with oseltamivir, hydroxychloroquine and low-molecular-weight heparin was initiated. The patient received supplemental oxygen through a nasal cannula at a rate of 4 l/min. Blood and urine cultures were negative.
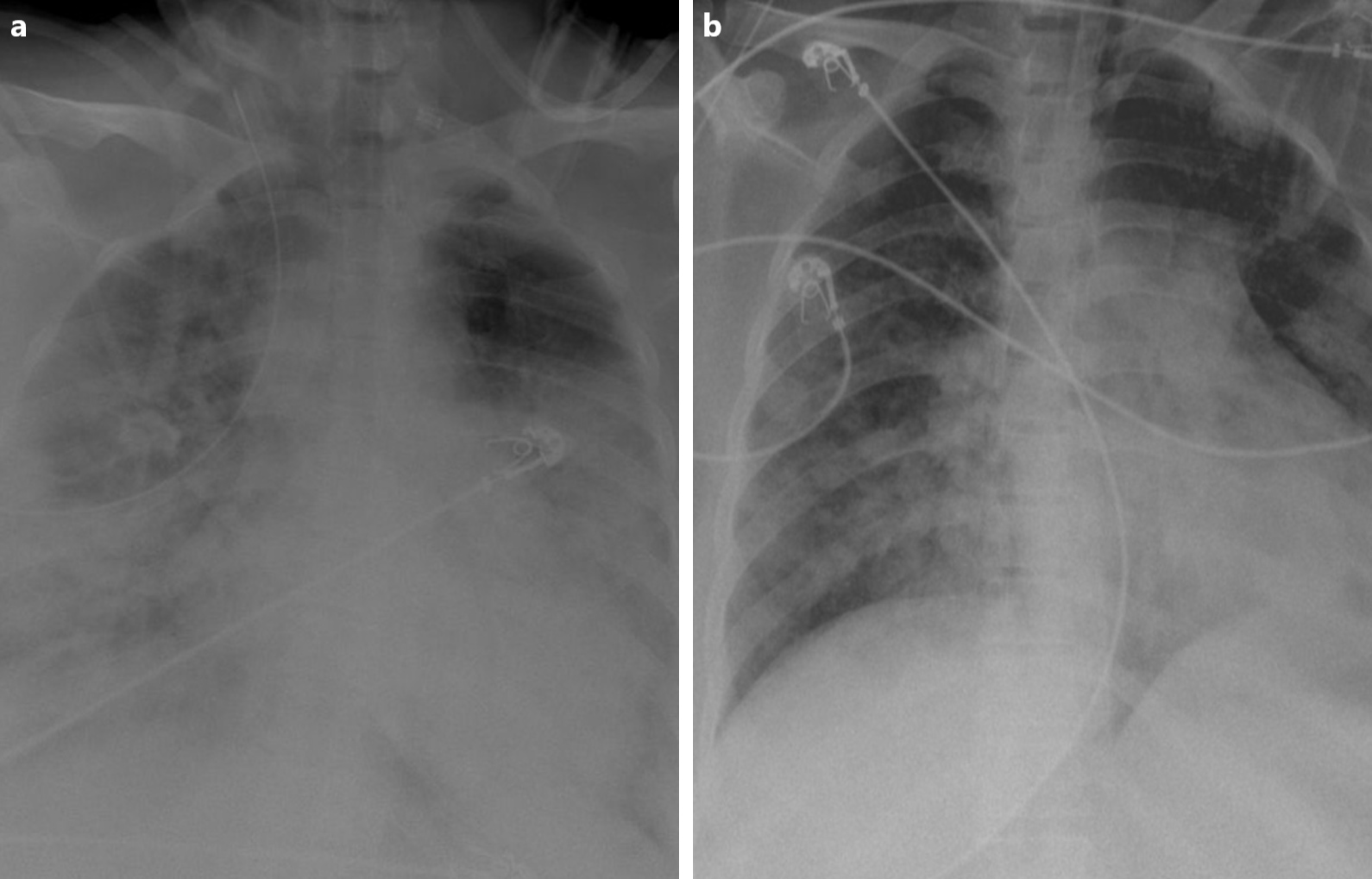
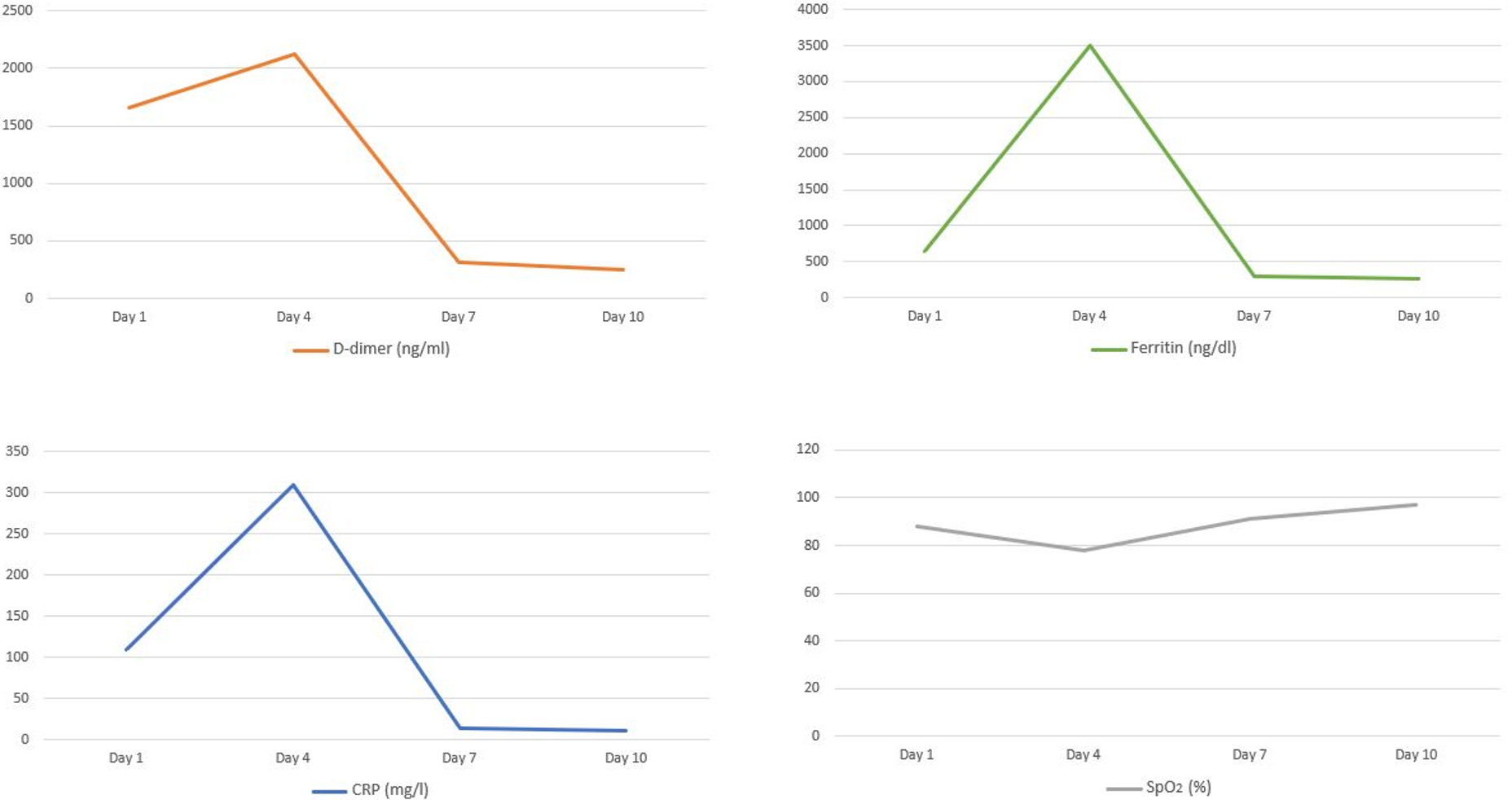
After 3 days of hospitalization the patient’s clinical condition deteriorated (respiratory rate of 26 breaths/min and SpO2 decreased to 78%) and she required intensive care unit (ICU) admission. A chest x-ray at the time of ICU admission revealed hazy bilateral lobe opacity (Fig. 2a). The patient was intubated and diagnosed with CRS. She was given a single intravenous (IV) dose of 400 mg TCZ and IV methylprednisone 60 mg daily for 3 days. Ventilatory support requirements reduced day-by-day. C-reactive protein, ferritin and D-dimer levels dropped from 310 mg/l, 3500 ng/dl and 2123 ng/ml on the day of ICU admission to 13.2 mg/l, 300 ng/dl and 320 ng/ml after 3 days, respectively. A chest x-ray showed that the lesions significantly improved within 3 days of TCZ administration (Fig. 2b). The patient was successfully extubated 5 days after treatment with TCZ. No adverse events were described. On day 10, a clear improvement in the patient’s general condition was observed, with an SpO2 of 97% without any need for supplemental oxygen (Fig. 3).
Figure 2. (a) X-ray of the lung, at the time of ICU admission, revealed hazy bilateral lobe opacity. (b) X-ray after treatment showed significant improvement
Figure 3. Laboratory and SpO2 data
DISCUSSION
COVID‐19 is associated with increased proinflammatory cytokines. The elevated cytokine levels may also be responsible for the lethal complications of COVID-19. Moreover, histopathological examination of lung tissue from deceased patients with severe COVID‐19 showed evidence of extensive alveolar oedema, proteinaceous exudate and patchy inflammatory cellular infiltration. These findings suggest that severe COVID‐19 infection is associated with a cytokine storm and pulmonary inflammation secondary to a dysregulated host immune response [3]. IL-6 contributes to host defence against infection; however, exaggerated synthesis of IL-6 while fighting environmental stress leads to an acute severe systemic inflammatory response. In the subset of patients with severe COVID‐19 infection, this cytokine activation presents with recognized features such as high plasma levels of C-reactive protein, D-dimer and ferritin, and a decreased lymphocyte count. TCZ is a monoclonal antibody against IL-6 and is currently approved to treat chronic inflammatory conditions such as rheumatoid arthritis, giant cell arteritis and polyarticular juvenile idiopathic arthritis [4]. CRS in a patient with COVID-19 is life-threatening and can take place very rapidly after COVID-19 diagnosis. Currently, there is no established treatment for COVID-19-associated CRS. TCZ is an option for use in the clinical management of COVID-19 patients with CRS and is associated with a dramatic improvement in inflammatory markers, radiological changes and hypo-oxygenemia.