ABSTRACT
Heparin is commonly used in clinical practice for the prevention and treatment of various thrombotic conditions. Its use can be associated with bleeding which can range from minor to life threatening. Non-traumatic causes of breast haematoma are very rare. We report a case of spontaneous bleeding into the breast in a female patient who was anticoagulated with heparin.
LEARNING POINTS
- Anticoagulant use can be associated with the adverse effect of bleeding at various sites.
- Physicians should be cautious of such bleeding which can occur at unsuspected sites.
- Our patient developed spontaneous breast haematoma after unfractionated heparin anticoagulation, and was successfully managed with cessation of anticoagulation, protamine, desmopressin and blood transfusion.
KEYWORDS
Thrombosis, heparin, anticoagulation, breast haematoma
INTRODUCTION
Heparin has been an important part of anticoagulation regimens available to physicians for many decades. While it has many prophylactic and therapeutic indications, its use can be associated with some clinically significant adverse effects. Haemorrhage is a well-recognized adverse effect of heparin use, even at usual therapeutic dose ranges. Usual sites of such bleeding are the skin, gastrointestinal tract, genitourinary tract, central nervous system, retroperitoneum, muscle, and at the site of recent surgical procedures or trauma. Breast haematomas are usually traumatic in origin. Spontaneous bleeding into the breast after anticoagulant use is a rare clinical entity. It results from extravasation of blood into the soft mammary gland tissue.
CASE DESCRIPTION
A 32-year-old African American woman with a medical history significant for end-stage renal disease (ESRD) secondary to systemic lupus erythematosus (SLE) received a deceased donor kidney transplant in May 2017. Her medical history also included hypertension and embolic stroke from non-infectious mitral valve vegetation for which she was on apixaban 5 mg twice daily. In June 2019 she was admitted to hospital after outpatient laboratory investigations showed elevated creatinine which was concerning for renal transplant rejection. She admitted to not being fully adherent with her immunosuppressant medications due to medical insurance issues. She reported a 3–4-day history of generalized body aches, nausea, right flank pain (particularly around the transplant site) and subjective fevers and chills. She denied any changes in urine output, haematuria or dysuria. Her baseline creatinine was 1.2 mg/dl, but on admission it was 5.3 mg/dl.
Her prescribed immunosuppression regimen consisted of azathioprine 50 mg daily, tacrolimus 5 mg twice daily and prednisone 5 mg daily. Admission laboratory tests showed a haemoglobin of 9.1 mg/dl and a tacrolimus level of 3.5 ng/ml. Apixaban was held at admission as a renal transplant biopsy was anticipated. The patient was not on any anti-platelet agent. The renal transplant ultrasound showed an increase in the size and echogenicity of the transplanted kidney with elevated resistive indices concerning for transplant rejection. There was no hydronephrosis and the renal transplant artery and vein were patent.
The patient’s creatinine failed to improve with intravenous hydration and worsened to 10.3 mg/dl. She underwent a renal transplant biopsy which showed severe acute cellular as well as antibody-mediated rejection. She also had high class II de novo donor-specific antibodies towards DQ7/DQA05 (25000 MFI). Due to worsening uraemia and plans for plasmapheresis (for antibody-mediated rejection), a right-sided internal jugular vein-tunnelled haemodialysis catheter was placed on day 6 and she was started on haemodialysis. Concurrently, she was being treated for renal allograft rejection with intravenous pulse steroids and thymoglobulin.
A day after dialysis catheter placement, the patient reported new onset facial swelling. Doppler ultrasound showed non-occlusive acute thrombus within the inferior aspect of the right internal jugular vein. Her INR was 1.0 and prothrombin time was 11.8 seconds. She was started on an intravenous unfractionated heparin drip with venous thrombosis protocol and the dialysis catheter was removed. Anti-Factor Xa levels were followed.
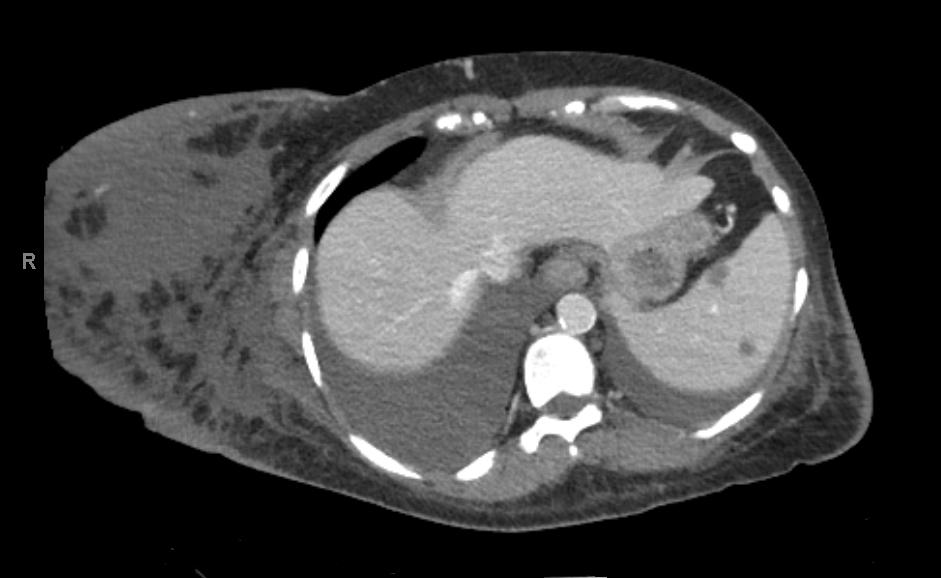
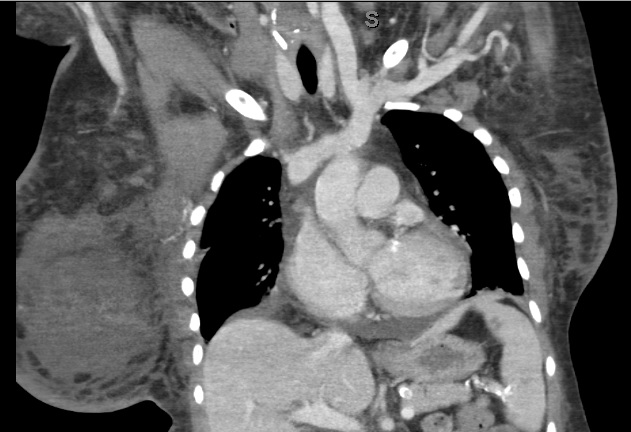
Three days later the patient complained of new onset right breast enlargement. Physical examination showed a significantly enlarged right breast with no erythema, ecchymosis or palpable mass. A new drop in haemoglobin to 5.4 mg/dl was also noticed on laboratory investigation. Her platelet count was 153,000 per mm3. While she was on the heparin drip, her anti-Factor Xa levels ranged between 0.11 and 0.87 IU/ml. The patient was tachycardic (heart rate 104 bpm) with stable blood pressure. Heparin anticoagulation was held. An emergent CT scan of the chest, abdomen and pelvis revealed a 7×11 cm right breast haematoma, with right pleural effusion greater than the left (Figs. 1 and 2) and a 3 cm circumferential transplant kidney perinephric haematoma without active bleeding.
Figure 1. CT scan (axial view) imaging showing a large right breast haematoma. Right and left pleural effusions are also seen
Figure 2. CT scan (coronal view) imaging showing a large right breast haematoma
At this point we administered protamine, desmopressin and 2 units of packed red blood cells. There was no overt bleeding from anywhere. The patient’s haemoglobin improved and tachycardia resolved, and she remained haemodynamically stable. The patient was advised to wear a support bra. A repeat CT scan 2 days later showed a decrease in the size of the right breast haematoma and stable perinephric haematoma. We continued haemodialysis support and treatment for renal transplant rejection. Another CT scan 28 days later for a different indication showed a further decrease in the size of haematoma.
DISCUSSION
Breast haematoma can result from traumatic or non-traumatic causes. Traumatic causes usually include sports injury or seat belt injury [1]. Iatrogenic causes include breast surgery and breast biopsy. Rarely, spontaneous bleeding into the breast can result from underlying coagulopathy, haematological disorders, use of anticoagulants, and rarely haemorrhage into a tumour [1, 2]. Breast haematoma may be asymptomatic or present as breast swelling, pain or discoloration of the skin of the breast. Small-sized haematomas usually resolve on their own due to blood resorption, but larger haematomas can lead to inflammation and fibrosis of breast tissue. Fat necrosis and seroma formation due to liquefaction of blood can occur. Diagnostic modalities include ultrasound, mammogram, CT scan, MRI and breast biopsy, depending upon the clinical scenario. Management usually involves supportive care, anticoagulation withdrawal, and correction of coagulopathy. Rarely, drainage may be needed [3, 4].
Spontaneous bleeding into the breast can occur due to anticoagulant and thrombolytic therapy [5–7]. Unfractionated heparin is widely used for the prevention and treatment of thromboembolism. Commonly encountered side effects include heparin-induced thrombocytopenia and haemorrhage at various sites. This case illustrates an unusual site of bleeding, most likely due to unfractionated heparin. A multipronged approach was used for the management of our patient. Heparin infusion was promptly discontinued, and protamine was used to reverse the heparin anticoagulant effect. The patient received blood transfusion and desmopressin was used given the high likelihood of an underlying platelet dysfunction due to uraemia. Given the widespread use of anticoagulants and thrombolytics, spontaneous bleeding into the breast should be considered in the right clinical setting. Due to the clinical context and improvement of haematoma with appropriate management, the possibility of tumour bleeding was considered highly unlikely in this case.