ABSTRACT
Tuberculosis (TB) is a cause of ill health and death worldwide. Since 2010, the diagnostic process has strongly relied on GeneXpert assays on biological specimens. Xpert MTB/RIF is an automated nucleic acid amplification test (NAAT) for Mycobacterium tuberculosis and rifampicin resistance, endorsed by the World Health Organization and the US Food and Drug Administration. Xpert is used in many countries as the initial diagnostic test for tuberculosis. Nevertheless, the reliability of GeneXpert positive tests in patients with a history of TB is largely unknown, due to possible false-positive results (i.e., GeneXpert-positive but culture-negative patients). We present a case report of a patient with a history of pulmonary TB, who was GeneXpert positive but culture negative on bronchoalveolar lavage 22 months after completion of appropriate antitubercular therapy.
LEARNING POINTS
- GeneXpert assays have a pivotal role in the diagnosis of tuberculosis (TB), with overall good sensitivity and excellent specificity.
- Patients with a history of TB can be GeneXpert positive up to several years after the end of appropriate antitubercular treatment.
- Further studies are warranted to fully understand the role of GeneXpert assays in the diagnostic algorithms of patients with a history of TB.
KEYWORDS
Tuberculosis, TB, GeneXpert, retested patients, false positives
INTRODUCTION
Tuberculosis (TB) is a communicable disease and a major cause of ill health. It is one of the top 10 causes of death worldwide and the leading cause of death from a single infectious agent (ranking above HIV/AIDS) [1]. The diagnosis of Mycobacterium tuberculosis (MTB) lung infection (pulmonary tuberculosis) is historically based on identification of the bacterium by direct examination or by culture of a respiratory sample. Since 2010, a specific polymerase chain reaction (PCR) assay, known as GeneXpert, has been used worldwide, as (a) in a controlled clinical validation trial it showed a cumulative sensitivity after three samples of 90.2%, (b) it shortened the time required to reach a diagnosis (providing results within 2 hours), and (c) permits quick identification of strains resistant to rifampicin (GeneXpert MTB/RIF specificity is 99%) [2–5]. If smear microscopy is positive, a positive PCR assay confirms that the organism is indeed MTB and not a non-tuberculous mycobacterium (NTM), since Xpert does not detect DNA from nontuberculous mycobacteria. However, this does not always indicate the presence of active disease caused by viable, intact bacilli, because of the inability to distinguish genomic DNA from nonintact cells, which may be present after successful treatment. On the other hand, if smear microscopy is negative, a positive PCR assay indicates the result of the colture will be positive, even though smear results will not be positive for another 2–6 weeks. For this reason it is recommended that Xpert always be used with culture, although it is not mandatory, depending on the national algorithms. After completion of appropriate treatment, the absolute risk of reinfection and/or recurrence of TB is about 5%, with half of these events occurring during the first year.
CASE DESCRIPTION
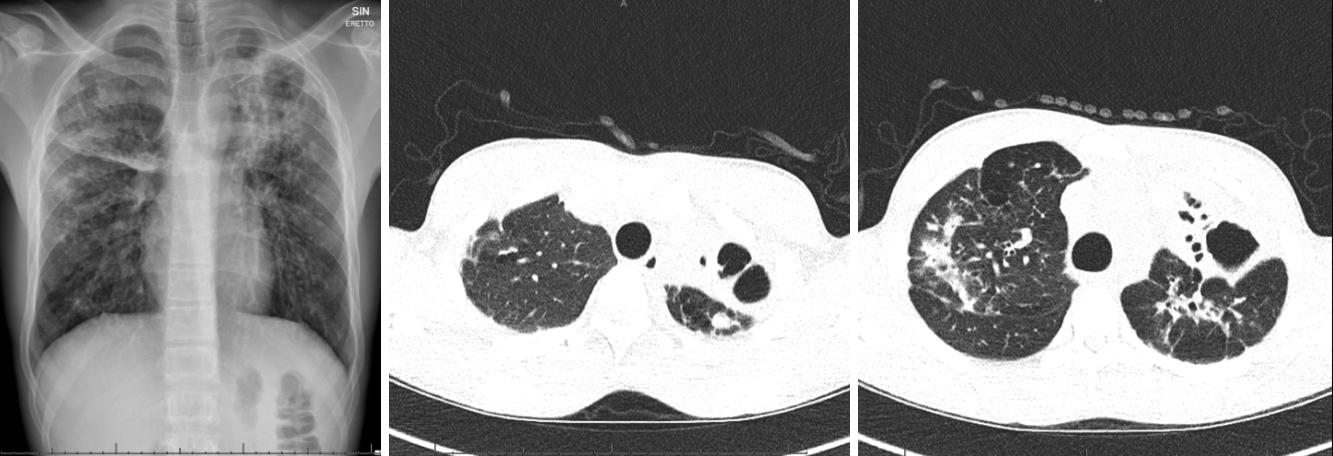
We present the case of an 18-year-old Somali patient who presented in December 2019 complaining of a 2-week history of asthenia and non-productive cough. His medical history was significant for pan-sensitive cavitary bacillary pulmonary tuberculosis, treated from June 2017 with rifampicin, isoniazid, pyrazinamide and ethambutol (INH, RIF, PZA, EMB) for 2 months, and with prolonged consolidation therapy (up to 8 months), due to pulmonary caverns, based on INH and RIF. Sputum cultures were negative from the third month of therapy. He took no other medications. On physical examination, the patient appeared pale and sick. Vital signs were normal. The patient’s cardiopulmonary status was unremarkable and there were no lymphadenopathies. Blood analysis showed a normal leukocyte count and C-reactive protein (CRP) of 48 mg/l. A rapid test for influenza A, B and respiratory syncytial virus was negative. Urinary antigen tests for Streptococcus pneumoniae and serotype 1 Legionella pneumophila were negative. Chest x-ray and CT findings were superimposable on previous scans, showing bilateral extensive bronchiectases with doubtful activity (Fig. 1).
Figure 1. Chest x-ray and chest CT scan showing extensive bilateral bronchiectases and bronchial thickening in a patient with a history of tubercular pneumonia
Due to the negative results of both direct examination and GeneXpert on sputum but the high epidemiological and clinical probability of tuberculosis, we performed bronchoalveolar lavage which was eventually GeneXpert positive but culture negative. After few days the patient was discharged from hospital. The clinical course was unremarkable, with rapid resolution of symptoms with no need for antibiotic therapy.
DISCUSSION
We present the case of a patient with a history of tubercular pneumonia, who tested GeneXpert positive but culture negative 22 months after the completion of a full course of antitubercular therapy. The clinical course, imaging, laboratory results and microbiology tests confirmed the false positivity of the GeneXpert assay. The false positivity may trigger unwarranted and unnecessary treatment with toxic drugs, and may cause the delay in establishing the correct diagnosis with its appropriate treatment, growing the healthcare costs. This case reveals that GeneXpert positivity can last almost 2 years after the completion of appropriate treatment. As of 2020, the role of GeneXpert assays in diagnosing reinfection or relapse has not yet been fully clarified. Theron et al. show that after the completion of appropriate treatment, GeneXpert can produce false-positive results for up to 4 years [4] and in 8.72% of positive tests on respiratory samples for up to 18 months [5], possibly due to both the presence of residual mycobacterial DNA in the respiratory tract and the detection of non-viable bacilli. In this context, the presence of cavitary lung lesions could contribute to false-positive results. The total duration of this false positivity is not yet fully known, so clinicians are not able to determine whether or not a positive GeneXpert result following well-treated pulmonary tuberculosis, signifies reinfection or recurrence. Previous studies have demonstrated a decreasing likelihood of GeneXpert false positivity the longer the time from appropriate treatment [5]. Nevertheless, there are no clear timing cut-offs and further studies are warranted to understand the role of this high-performing test in the diagnostic algorithms of patients with a history of TB. In conclusion, GeneXpert tests significantly shorten the time to diagnosis of M. tuberculosis infection. Nevertheless, due to false-positive results, its role in diagnosing reinfection or relapse is not fully understood and further data are needed to fully clarify its utility in this setting.