ABSTRACT
We describe the case of a male patient admitted to our emergency department during the Italian COVID-19 epidemic, for progressive worsening dyspnoea. A diagnosis of pneumothorax and diffuse interstitial lung involvement was promptly made by lung ultrasound and confirmed by an HRCT scan. A chest CT scan also showed diffuse emphysema, as observed in chronic obstructive pulmonary disease (COPD), and small consolidations in the lower lobes, suggestive for COVID-19 pneumonia. A chest tube was immediately inserted in the emergency room with complete resolution of the dyspnoea. A nasopharyngeal swab for 2019-nCoV was positive. Unfortunately, the patient died from COVID-19-related acute respiratory distress syndrome after 48 days of hospitalization.
LEARNING POINTS
- Coronavirus disease (COVID-19) can cause death from severe acute respiratory distress syndrome (ARDS).
- Pneumothorax is a common complication of chronic obstructive pulmonary disease (COPD).
- The combination of COVID-19, COPD and pneumothorax can prove fatal.
KEYWORDS
COVID-19, pneumonia, dyspnoea, pneumothorax, COPD, ARDS
INTRODUCTION
Coronavirus disease 2019 (COVID-19), also called SARS-CoV-2, is caused by 2019 n-CoV, a new type of coronavirus first identified in China at the end of December 2019. It rapidly spread worldwide, causing significant morbidity and mortality, and developed into a global pandemic. Acute respiratory distress syndrome (ARDS) is the most common complication of COVID-19, and the main cause of death. We present the case of an 87-year-old man with a diagnosis of chronic obstructive pulmonary disease (COPD) and COVID-19 pneumonia complicated by pneumothorax.
CASE DESCRIPTION
We report the case of an 87-year-old male patient who was admitted to our emergency department during the COVID-19 epidemic, with a 6-day history of worsening dyspnoea without cough or fever. He was a heavy smoker. He had never undergone pulmonary[Q2] evaluation. He was taking low-dose aspirin for primary prevention of cardiovascular disease, as prescribed by his general practitioner.
At admission the patient denied previous contact with any individual positive for SARS-CoV-2. He was tachypnoeic (28 breaths/minute) and dyspnoeic (SpO2 93% with FiO2 40%). Arterial blood gas (ABG) analysis, blood tests and nasopharyngeal swabs for 2019-nCoV, an electrocardiogram (EKG) and point-of-care lung ultrasound (LUS) were carried out. ABG analysis showed severe hypoxaemia (PaO2/FiO2 180 mmHg) with compensated metabolic acidosis (pH 7.30, PaCO2 35 mmHg, HCO3 20). EKG showed sinus tachycardia. Blood tests demonstrated lymphocytopenia (1330/mm3, normal value 1500–4000), increased C-reactive protein (2.85 mg/dl, normal value 0–0.5), creatinine (1.52 mg/dl, normal value 0.6–1.2) and urea (94 mg/dl, normal value 10–50) with normal serum electrolytes, lactate dehydrogenase, liver function and coagulation times.
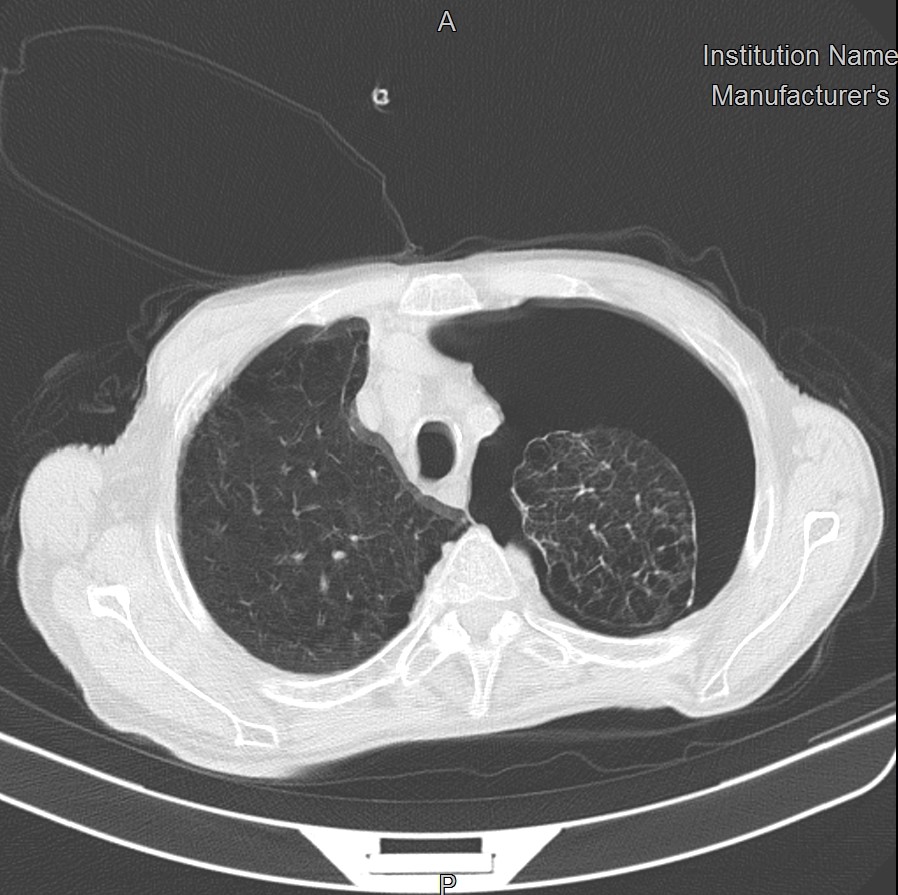
LUS revealed the absence of lung sliding and the loss of B lines in the upper left lobe (‘supine pneumothorax’) and diffuse B lines with irregular pleural thickening in the posterior part of both lobes. High-resolution chest CT scan (HRCT) confirmed the diagnosis of left pneumothorax and revealed diffuse emphysema, as observed in chronic obstructive pulmonary disease (COPD), and small consolidations in both lower lobes, suggestive of COVID-19 pneumonia (Fig. 1).
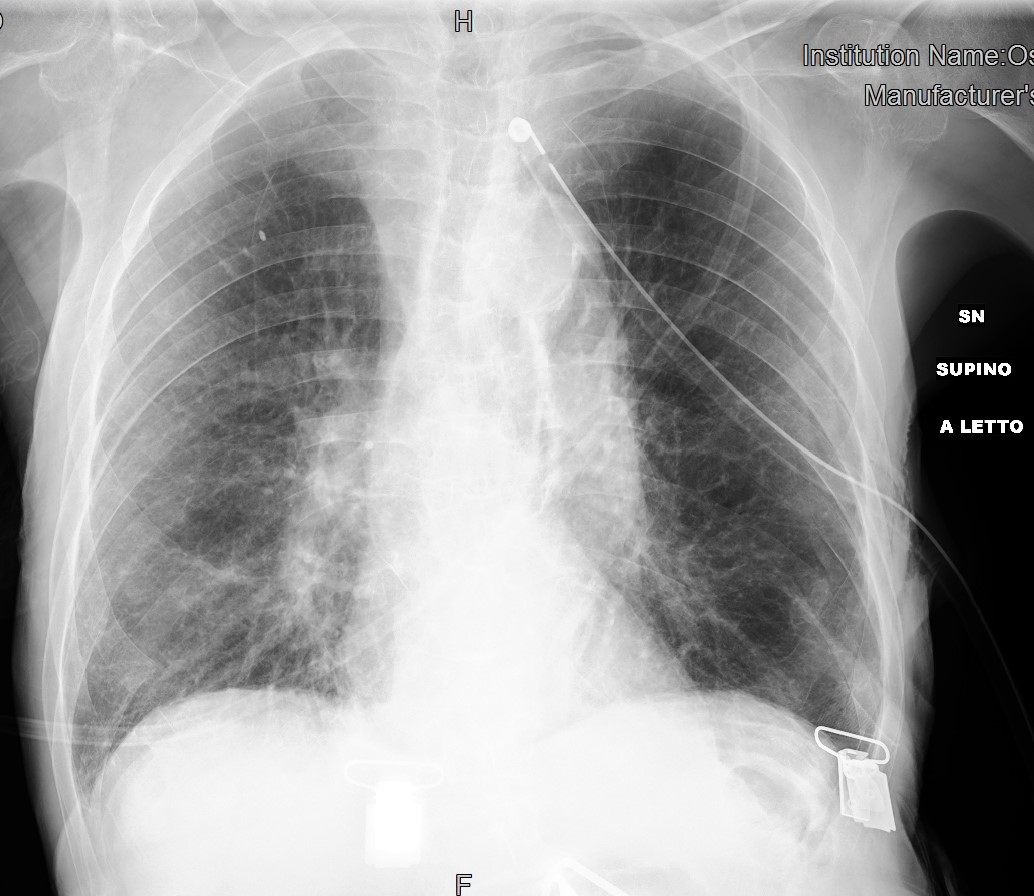
A chest tube for drainage was immediately inserted in the emergency room with complete resolution of the dyspnoea and pneumothorax on chest x-ray (CXR) (Fig. 2).
Figure 1. HRCT of the chest at admission showing a left pneumothorax with complete collapse of the unilateral pulmonary lobe, and diffuse paraseptal, centrilobular and panlobular emphysema with ground-glass opacities and small consolidations in the right lobe
Figure 2. Chest x-ray after chest tube placement in the emergency room showing complete resolution of the pneumothorax and evidence of small multiple consolidations in the right middle-lower lobe
The patient was diagnosed with atraumatic pneumothorax and COPD related to suspected COVID-19 pneumonia. A nasopharyngeal swab for 2019-nCoV was positive, confirming the diagnosis of COVID-19 pneumonia. The patient was treated with darunavir/cobicistat, hydroxychloroquine and a systemic steroid. Pulmonary evaluation confirmed the diagnosis of COPD.
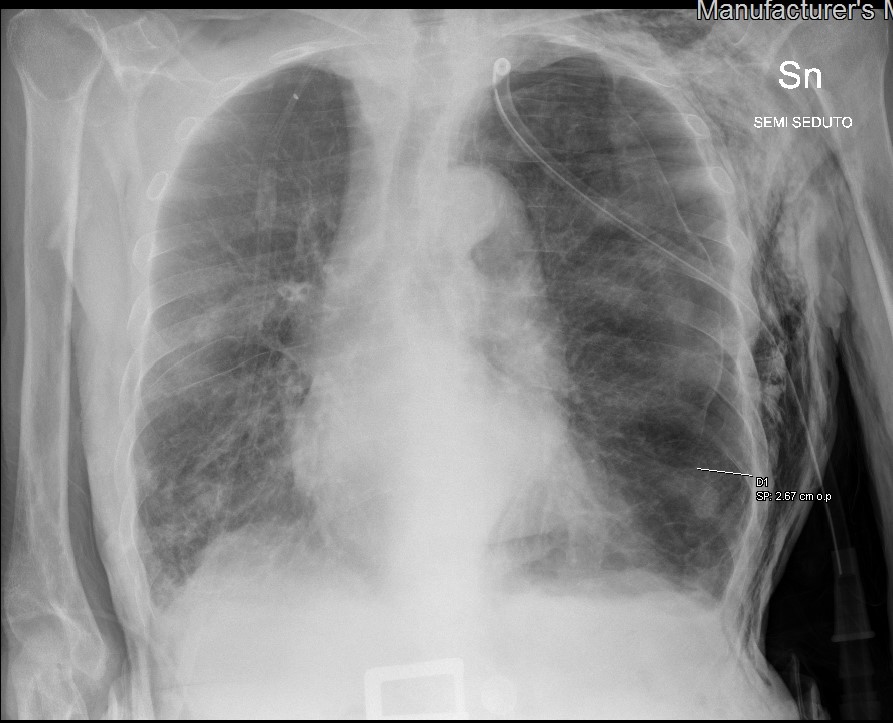
After 3 days of hospitalization, the patient complained of acute dyspnoea while the chest tube was closed. CXR showed recurrence of a left pneumothorax, and an increase in the size and number of consolidations in the lower lobes, suggesting progression of COVID-19 pneumonia (Fig. 3).
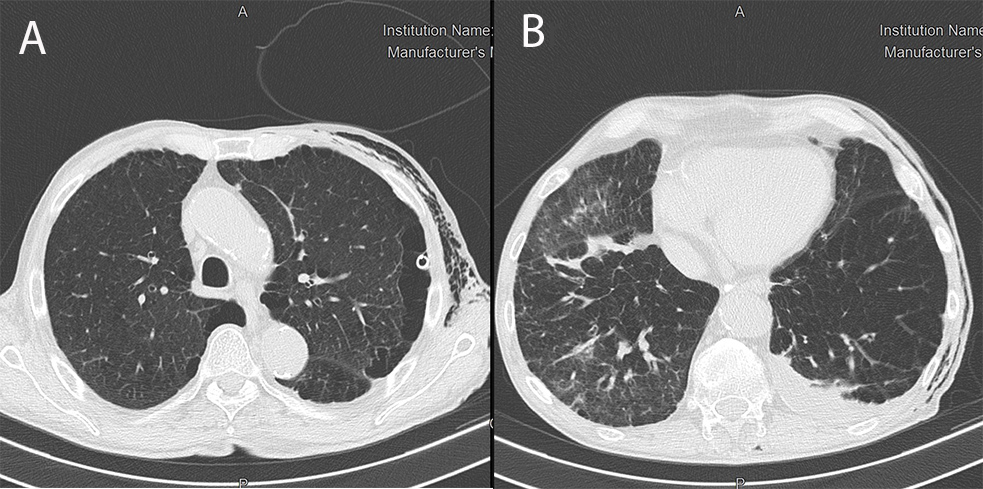
The patient refused talc pleurodesis. Surgical pleurodesis was not indicated because of the high risk of complications due to COPD and COVID-19. The pneumothorax completely solved with chest tube reopening, but respiratory distress progressively increased. We repeated HRCT, which demonstrated complete resolution of the left pneumothorax (Fig. 4A), but worsening of COVID-19 pneumonia (Fig. 4B). The patient refused all therapeutic options, including intubation. A psychiatric evaluation excluded a mental disorder. The chest drain was removed with no further recurrence. The patient died 48 days after admission from COVID-19-related ARDS (Fig. 5).
Figure 3. Chest x-ray showing recurrence of the left pneumothorax with a closed chest tube, and the worsening of multiple small consolidations in both pulmonary lobes
Figure 4. HRCT showing complete resolution of the left pneumothorax (A) and worsening of COVID-19 pneumonia with ground-glass opacities, a crazy-paving pattern and diffuse small consolidations in both pulmonary lobes (B)
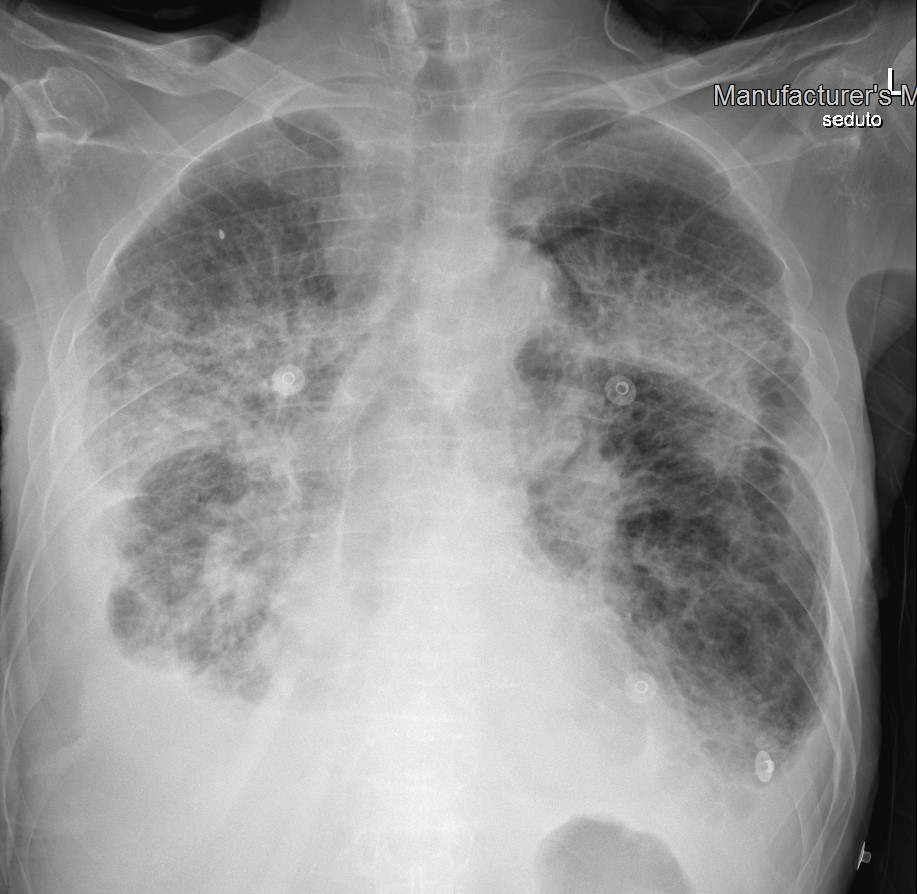
Figure 5. Chest x-ray showing bilateral, diffuse lung opacities involving all pulmonary quadrants
DISCUSSION
Pneumothorax can be due to lung disease, particularly COPD with emphysema, and is far more common in men than in women. Risks factors for pneumothorax include smoking, genetics, lung disease, mechanical ventilation and previous pneumothorax. The diagnosis of pneumothorax is usually based on a combination of clinical signs and symptoms, and plain CXR. Point-of-care LUS is the gold standard technique to diagnose pneumothorax in the emergency and critical care setting. Sonographic signs of pneumothorax are the absence of lung sliding and B lines [1]. Ultrasound has a higher sensitivity than the traditional CXR for the detection of pneumothorax [2, 3]. Pneumothorax is treated with needle aspiration and catheter drainage guided by ultrasound. In case of intractable or recurrent pneumothorax, pleurodesis can be considered. Chemical pleurodesis is indicated for inoperable patients. The complications of pneumothorax include effusion, haemorrhage and empyema. Respiratory failure, pneumomediastinum and arrhythmias can also occur. Smokers are more likely to relapse and are prone to the complications and sequelae. Patients with COPD, bronchiectasis, tuberculosis, cystic fibrosis and interstitial lung disease should be treated and followed more carefully [4].
COPD is a chronic inflammatory process of the airways, lung parenchyma and pulmonary vasculature, which can lead to abnormal permanent enlargement of the air space distal to the terminal bronchioles, accompanied by destruction of the alveolar walls, with the development of pulmonary emphysema [5]. Lung inflammation can be caused by cigarette smoke, environmental pollutants or bacterial products. HRCT has a pivotal role in the diagnosis of pulmonary emphysema [6].
COVID-19 is a systemic infectious disease caused by the novel coronavirus, 2019-nCoV. COVID-19 can cause respiratory symptoms ranging from a flu-like syndrome to ARDS with death due to multi-organ failure [7, 8]. As we have recently published, LUS can be useful for the diagnosis of COVID-19 pneumonia in the emergency room [9].
Human coronaviruses are the second most common cause of viral pneumonia in COPD patients, after influenza virus [10]. Mediastinal emphysema, giant bulla and pneumothorax have been observed during the course of COVID-19 [11].
CONCLUSION
COVID-19 pneumonia is characterized by alveolar swelling, a small amount of exudate in the alveolar space, and alveolar septal inflammation. Progression to ARDS causes diffuse alveolar injury and a pro-inflammatory cytokine storm, which can induce alveolar rupture [12]. Based on these observations, patients with COPD and pulmonary emphysema are at high risk of pneumothorax when infected with 2019-nCoV. Special attention should be paid to these patients. Early imaging diagnosis based on the combination of LUS and HRCT, and timely treatment of COVID-19 complications, including pneumothorax, are paramount to improve the prognosis of COVID-19 patients and to reduce the risk of COVID-19-related ARDS.