ABSTRACT
An 81-year-old man complaining of exertional dyspnoea underwent coronary angiography using an iodinated contrast medium. After angiography, the patient required systemic corticosteroid therapy because of respiratory failure due to alveolar haemorrhage. Percutaneous coronary intervention was performed 29 days after angiography using the same contrast medium. After the intervention, the patient required intubated mechanical ventilation and renal replacement therapy. Bronchoalveolar lavage was bloody with many haemosiderin-filled macrophages. Systemic corticosteroid therapy again improved his clinical condition. Iodinated contrast media may cause alveolar haemorrhage and re-exposure to contrast media may induce a more severe adverse reaction.
LEARNING POINTS
- Iodinated contrast media may cause alveolar haemorrhage.
- Re-exposure to iodinated contrast media may induce a more severe adverse reaction.
KEYWORDS
Iodinated contrast media, alveolar haemorrhage, re-exposure
INTRODUCTION
Iodinated contrast media (ICM) are used in daily clinical practice, and occasionally induce adverse reactions including diffuse oedema, hypotension, bronchospasm and nephrotoxicity [1]. We encountered a rare adverse phenomenon, alveolar haemorrhage caused by ICM use and requiring intubated mechanical ventilation and renal replacement therapy.
CASE DESCRIPTION
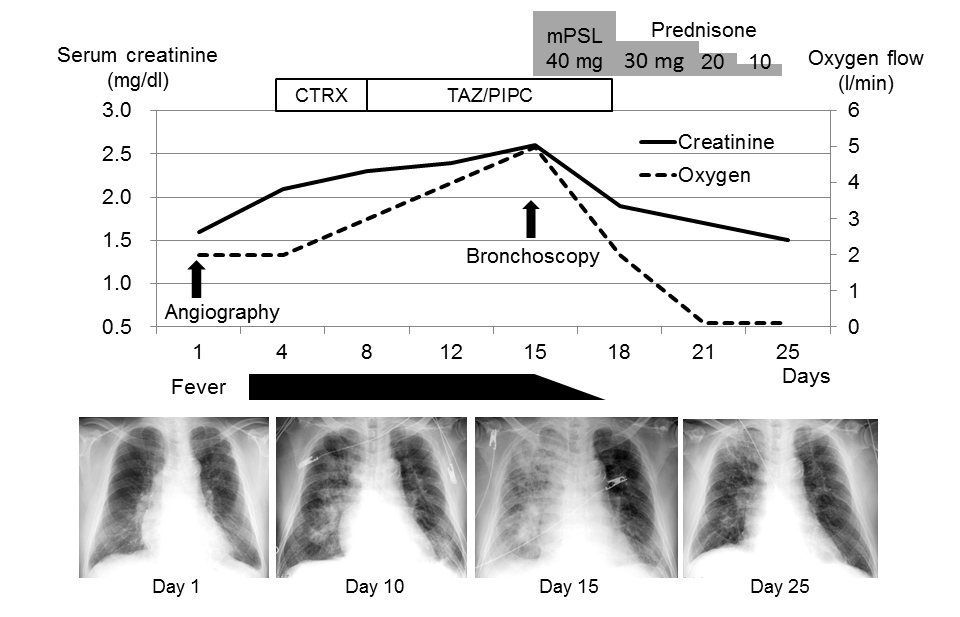
An 81-year-old man presented to the outpatient department complaining of exertional dyspnoea. Both legs were swollen with pitting oedema. He had chronic kidney disease due to hypertension, and a serum creatinine level on admission of 1.6 mg/dl without microscopic haematuria. On the first day of admission, coronary angiography was performed using 18 ml of ICM (Omnipaque 350 injection, Daiichi Sankyo Company Limited, Tokyo, Japan), revealing chronic total occlusion of the right coronary artery. Echocardiography showed diffuse hypokinesis of the left ventricle with an ejection fraction of 35% and enlargement of the inferior vena cava without respiratory collapse. Intravenous vasodilator and diuretic agents were administered for congestive heart failure. The patient’s weight rapidly decreased as the leg oedema resolved. However, the patient had fever with right-sided predominant consolidations on chest radiographs (Fig. 1).
Figure 1. Clinical course and changes in radiographic findings from admission to percutaneous coronary intervention.
CTRX, ceftriaxone; mPSL, methylprednisolone; TAZ/PIPC, piperacillin-tazobactam
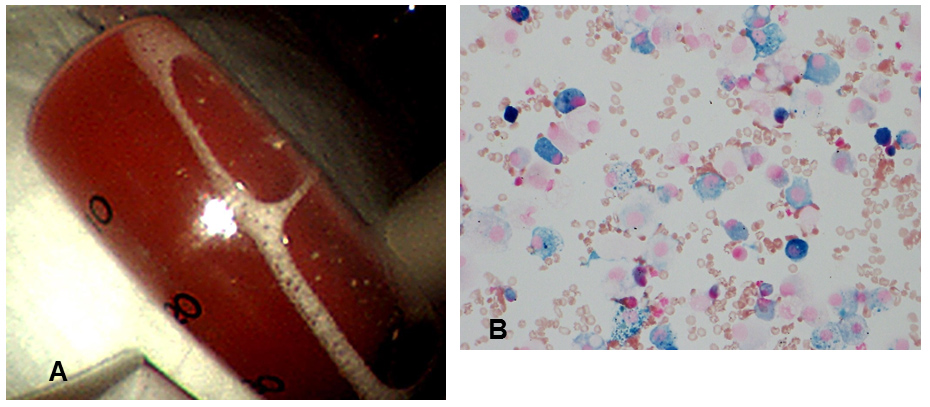
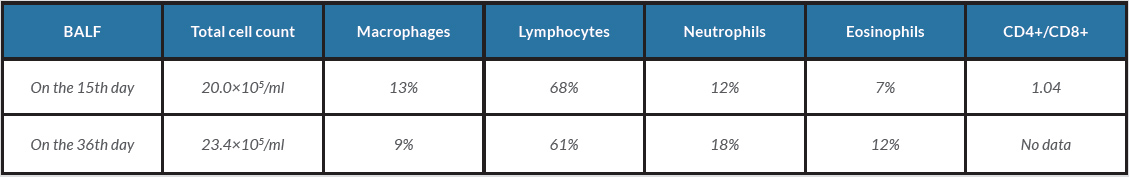
Ceftriaxone and piperacillin-tazobactam were administered, but expanded consolidations seen on subsequent chest radiography prompted the administration of high-flow oxygen therapy. The serum creatinine level increased to 2.6 mg/dl with microscopic haematuria (20–30 cells per high-power field). Echocardiography on the 14th day of admission showed a normal-sized inferior vena cava with respiratory collapse and no valvular abnormalities. On the 15th day of admission, bronchoalveolar lavage of the right middle lobe (S5a) revealed bloody fluid and hemosiderin-filled macrophages (Fig. 2A,B). The differential cell counts in the fluid showed lymphocyte predominance (Table 1).
Serum autoantibody tests, including anti-neutrophil cytoplasmic antibody, were all negative. After the initiation of systemic corticosteroid therapy with 40 mg of intravenous methylprednisolone daily, the patient’s general condition, including renal function and chest radiographic findings, improved. On the 25th day of admission, systemic corticosteroid therapy was discontinued.
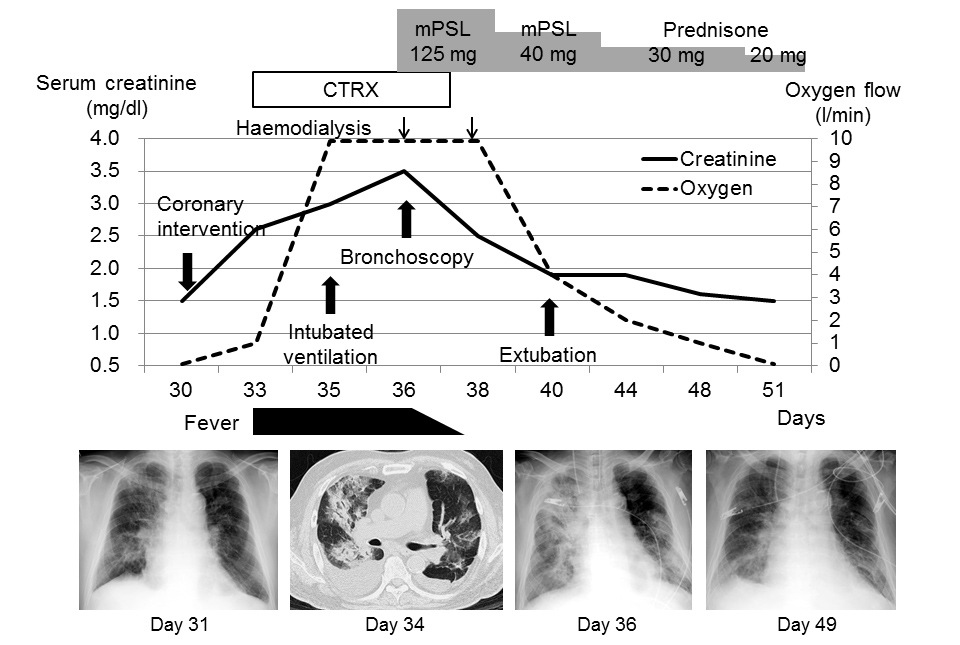
On the 30th day of admission, percutaneous coronary intervention for the occluded right coronary artery was performed using 45 ml of ICM (Omnipaque 350 injection). Echocardiography on the following day showed improvement of left ventricular wall motion with an ejection fraction of 47%, a normal-sized inferior vena cava with respiratory collapse, and no valvular abnormalities. However, respiratory function deteriorated and fever developed. Renal function also deteriorated with gross haematuria (>100 cells per high-power field) developing. The patient required intubated mechanical ventilation with chest radiographs showing consolidations on both lung fields, and renal replacement therapy was initiated (Fig. 3).
Figure 2. (A) Bronchoalveolar lavage on the 15th day of admission showing bloody fluid from the right middle lobe. (B) Iron staining of bronchoalveolar lavage fluid showing hemosiderin-filled macrophages (400× magnification)
Figure 3. Clinical course and changes in radiographic findings after percutaneous coronary intervention.
CTRX, ceftriaxone; mPSL, methylprednisolone
Bronchoalveolar lavage of the right middle lobe (S5a) retrieved bloody fluid with 49% of macrophages filled with hemosiderin. The patient’s condition improved after the administration of 125 mg of intravenous methylprednisolone daily. He was extubated following better radiographic findings, and renal replacement therapy was discontinued following improved renal function.
DISCUSSION
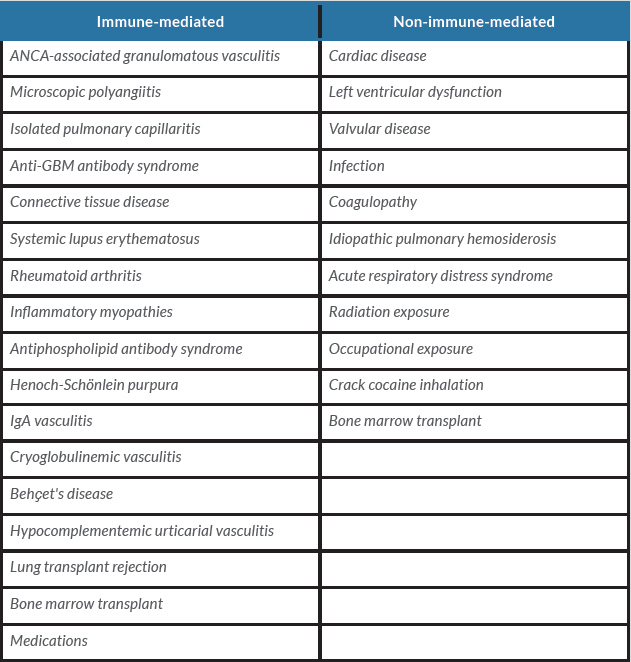
We encountered a case of alveolar haemorrhage with acute kidney injury induced by ICM use. ICM occasionally cause adverse reactions, which are classified as physiological reactions, nephrotoxicity and hypersensitivity reactions [1, 2]. Physiological reactions, such as nausea, headache and flushing, can be caused by ICM chemotoxicity, and are mostly self-limiting and rarely require therapeutic intervention [1]. Nephrotoxicity is caused by direct tubular injury due to contrast molecule-specific chemotoxicity [3]. Acute kidney injury occurred in 12% of patients undergoing percutaneous coronary intervention [4]. Hypersensitivity reactions to ICM cause urticaria, erythema, facial oedema, hypotension and bronchospasm [1]. To the best of our knowledge, there are no previous reports of an association between ICM use and alveolar haemorrhage. Therefore, in the present case, we did not realize the relationship until the second deterioration occurred after percutaneous coronary intervention. The aetiology of alveolar haemorrhage can be divided into immune-mediated and non-immune-mediated (Table 2) causes [5].
Table 2. Differential diagnosis of diffuse alveolar haemorrhage
ANCA, anti-neutrophilic cytoplasmic autoantibodies; GBM, glomerular basement membrane.
Our patient showed a marked decrease in body weight and resolution of leg oedema in response to treatment for congestive heart failure. Echocardiographic findings also revealed improvement in heart failure and no valvular abnormalities. Laboratory coagulation tests showed no abnormal findings, and the observed alveolar haemorrhage resolved without cessation of antiplatelet agent administration after corticosteroid therapy. Neither bronchoalveolar lavage sample showed significant microorganisms, and administration of intravenous antibiotics did not result in improvement. Furthermore, the present case showed that immunosuppressive therapy with systemic corticosteroid improved alveolar haemorrhage. Therefore, the observed alveolar haemorrhage could have been immune-mediated.
The first and second alveolar haemorrhages both occurred after administration of the same ICM. Some studies have indicated that ICM use affects the human immune system. Fanning et al. reported neutrophil apoptosis induced by ICM use, which could damage host immune defence and hinder resolution of the inflammatory response [6]. A study including 37 participants showed that coronary angiography using ICM caused significant elevation of serum inflammatory biomarkers such as interleukin-6 and tumour necrosis factor alfa [7]. Moreover, ICM use was reported to increase vascular permeability by inhibiting endothelial nitric oxide production [8]. In the present case, a remarkable increase in lymphocyte count was observed in bronchoalveolar lavage fluid, and haematuria was accompanied by alveolar haemorrhage. Hence, the observed alveolar haemorrhage might have resulted from inflammation and capillary hyper-permeability caused by ICM use. Unfortunately, we were unable to demonstrate the exact mechanism of alveolar haemorrhage because a histological specimen could not be obtained due to severe respiratory failure and the requirement for antiplatelet agent.
Our patient showed more intense adverse reactions following re-exposure to ICM. T lymphocyte activation is an important part of the hypersensitivity reaction to ICM use, and these activated T lymphocytes persist in peripheral tissues as memory T lymphocytes [9, 10]. Upon re-exposure to the same antigen, these memory T lymphocytes could provoke a more intense reaction compared to the initial response [11]. Kim et al. demonstrated that repeated ICM use was a significant risk factor for severe hypersensitivity reaction [12]. Premedication using corticosteroid and an anti-histamine is common to prevent recurrent hypersensitivity reaction. However, premedication alone is not sufficient and some recent studies have demonstrated that a combination of premedication and ICM alteration is the best strategy [13, 14].
In conclusion, ICM use can cause alveolar haemorrhage provoked by inflammation and capillary hyper-permeability. Moreover, re-exposure to ICM might induce a more severe reaction compared with the initial response.