ABSTRACT
We describe long-lasting and incompletely resolved thrombocytopenia after transient profound pancytopenia in a 62-year-old female patient with glioblastoma after short-term radiotherapy with temozolomide. Pancytopenia was present for more than 4 weeks and thrombocytopenia for more than 6 months, without platelet recovery to normal levels.
LEARNING POINTS
- Some patients may experience severe haematological manifestations after even short-term radiotherapy with temozolomide.
- In everyday practice, clinical models precisely predicting the haematological toxicity of concomitant treatment with temozolomide and radiotherapy is necessary, especially in countries where genetic tests are not available.
- In the primary care setting, misdiagnosis of a patient with spontaneous cerebral haemorrhage with chest discomfort suggesting cardiac aetiology and prompting antiplatelet/aspirin therapy could result in disaster.
- TIncomplete recovery of the cells of a particular bloodline over a long period may necessitate permanent discontinuation of chemotherapy or radiotherapy.
KEYWORDS
Glioblastoma, temozolomide, pancytopenia, thrombocytopenia
INTRODUCTION
The methylation status of the O6-methylguanine-DNA-methyltransferase (MGMT) promotor in glioblastoma may have prognostic and predictive value regarding response to therapy with temozolomide [1]. Temozolomide is frequently used to treat malignant gliomas, and many of the side-effects attributed to this drug may in fact be due to the underlying disease. However, Altinoz et al. described a case of pancytopenia in a 46-year-old female patient with glioblastoma associated with temozolomide treatment, which may have been related to a variant of the MGMT gene [2]. We report a similar case of pancytopenia and persistent thrombocytopenia in a patient with glioblastoma treated with temozolomide and radiation. Unfortunately, we were unable to examine the patient's MGMT gene.
CASE DESCRIPTION
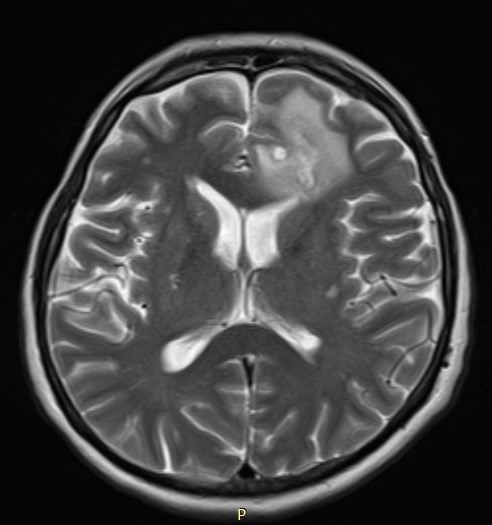
Six weeks after maximum safe resection of glioblastoma, in April 2016, a 62-year-old female patient with glioblastoma was admitted to our hospital for further treatment. The control MRI scan showed the presence of a residual tumour in the frontal lobe (Fig. 1). New surgical treatment was not considered at that time.
Figure 1. Contrast-enhanced T2-weighted MRI showing left frontal residual glioblastoma and peritumoral oedema
External beam radiotherapy was planned with three-dimensional conformal radiotherapy (RT) to a dose of 60 Gy in 30 fractions for 6 weeks with a concomitant daily dose of temozolomide (TMZ) of 140 mg (75 mg/m2). At the time, the patient’s baseline blood counts were almost normal: white blood cells (WBC) 3.8×109/l, platelets (Plt) 151×109/l and haemoglobin (Hb) 126 g/l, with normal standard serum chemistry. After 26 Gy in 13 fractions of RT concurrent with 21 days of TMZ, the patient developed diarrhoea and fatigue (both grade 1), and Clostridium difficile gut colonization was confirmed in faeces. The combined treatment was suspended.
Two weeks after completing treatment with oral metronidazole, the patient was admitted in order to continue RT with TMZ. At admission, her blood counts revealed severe pancytopenia (Hb 118 g/l, WBC 0.9×109/l, absolute neutrophil count (ANC) 0.3×109/l and Plt 4×109/l; all grade 4 except Hb, according to Common Terminology Criteria for Adverse Events, version 4 criteria). A few hours later the patient developed petechial bleeding in the skin and became subfebrile. She received platelet transfusions and ceftazidime and filgrastim 48 MIU was started. Anticonvulsants were discontinued. After 5 days of filgrastim treatment there was a gradual recovery of WBC and ANC counts, but without improvement in thrombocytopenia (grade 4). The haemoglobin level gradually declined, with a nadir of 67 g/l. The patient received a total of nine platelet transfusions, and one transfusion of PRBC. However, despite the transfusions, platelets remained low (the maximum recorded was 47×109/l).
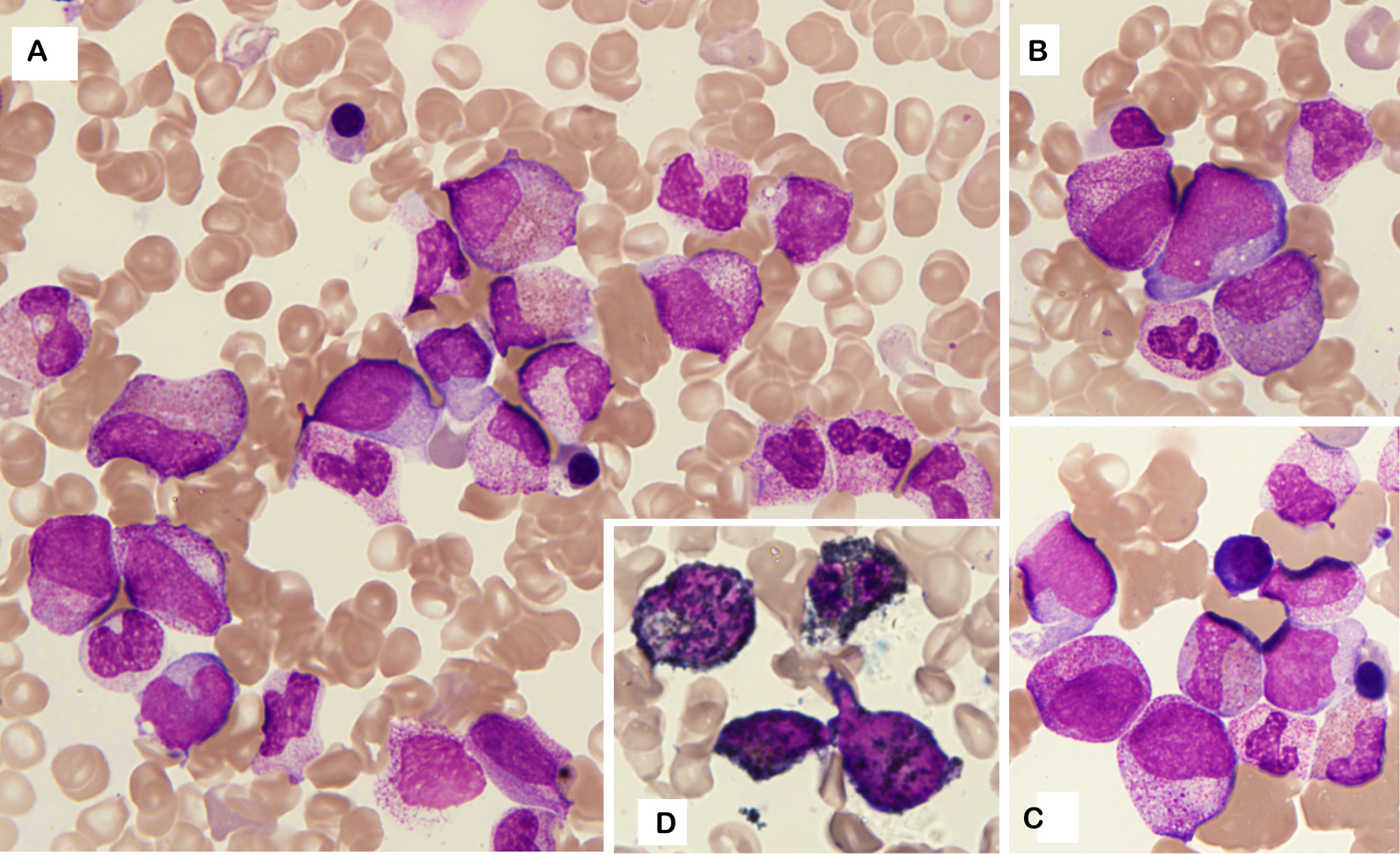
A complete haematological work-up was performed. Bone marrow aspiration (Fig. 2A) revealed a normocellular bone marrow with the presence of blast cells (8%) with occasional promyelocytes (5%) (Fig. 2B,C) but without dysplasia and with abundance of eosinophils (15%) with left shift. Megakaryocytes were almost absent. Blasts were myeloperoxidase positive (Fig. 2D). Due to the presence of blasts and promyelocytes and immature eosinophils, immunophenotyping of the bone marrow was performed and showed no signs of leukaemia but demonstrated features related to myeloid recovery. The karyotype was normal as well. Bone marrow core biopsy did not show any signs of leukaemia or profound aplasia.
Other diagnostic tests performed in order to evaluate long-term thrombocytopenia (Coombs test, vitamin B12 and folic acid levels, HBV, HCV and HIV) were unremarkable. There was no hepatosplenomegaly on ultrasound.
Figure 2. Bone marrow aspirate (May–Grünwald–Giemsa, 1000×). (A) Normocellular bone marrow with abundance of eosinophils with left shift and occasional blast cells. (B, C) Details revealing blast cells and promyelocytes. (D) Myeloperoxidase staining of blast cells
Thrombocytopenia persisted for the next 6 months, ranging from 40 to 60×109/l. In December 2016, a control MRI revealed tumour progression. The patient’s blood counts still showed decreased platelet levels (94×109/l) with slight to moderate neutropenia (ANC 1.4×109/l). The patient underwent a second surgical tumour resection in January 2017, and unfortunately died due to progressive disease in June 2017. The family refused a post-mortem.
DISCUSSION
Myelosuppression and myelodysplasia can be seen in patients treated with alkylating agents, but prolonged myelosuppression or suppression of a particular blood cell line is rare [3, 4]. In a retrospective study that included 680 patients, Armstrong et al. identified several risk factors that could predict the development of severe myelotoxicity during the first cycle of adjuvant TMZ treatment, although none of those patients had been treated concurrently with RT [5]. Although described as a risk factor in men, our female patient was treated for intestinal infection caused by C. difficile. Vandraas et al. reported the case of a patient who developed severe bone marrow failure after 3 weeks of concurrent RT–TMZ resulting in prolonged pancytopenia for 12 months [6]. In our patient, pancytopenia was present for more than 4 weeks and thrombocytopenia for more than 6 months, without platelet recovery to normal levels. Several reports pointed out that anticonvulsant treatment such as valproic acid (VPA) or levetiracetam (LEV) can induce haematological side-effects on their own [7]. In relation to antiepileptic drugs that patients took during concomitant RT–TMZ treatment, Tinchon et al. analysed the behaviour of and trends in Hb level, ANC, lymphocyte and platelet counts. They found that all parameters followed decreased over time in all three groups (VPA, LEV or without antiepileptic drugs), but without significant differences among them [7]. Soon after pancytopenia was diagnosed in our patient, VPA was excluded, but there was no significant improvement in blood counts except for the direct effect due to filgrastim. Data about possible drug-induced thrombocytopenia caused by metronidazole is provided by some authors [8]. However, in addition to long-term thrombocytopenia, our patient also had a pancytopenia for more than 4 weeks.
Although there may be genetic predispositions that cannot be influenced, a clinical model for predicting haematological toxicity may be improved by collecting enough data through bigger prospective and retrospective studies. Further genetic research is encouraged.