ABSTRACT
Background: Non-bacterial thrombotic endocarditis (NBTE) is a paraneoplastic phenomenon with sterile vegetations. It is associated with adenocarcinoma and can shower emboli, which can be the presenting symptom.
Case Presentation: A 44-year-old woman with adenocarcinoma of the lung presented with chest pain, left hand weakness, and ataxia due to repeated embolic showering from NBTE to the central nervous system (CNS) and spleen.
Conclusion: NBTE is a rare condition that should be on the differential diagnosis in patients with culture-negative endocarditis and a history of adenocarcinoma.
LEARNING POINTS
- Differentiating non-bacterial thrombotic endocarditis (NBTE) from infective endocarditis can be a diagnostic challenge due to slow growing organisms; laboratory findings that suggest NBTE include the lack of leucocytosis, normal C-reactive protein, negative blood culture sets, and positive antiphospholipid antibodies.
- Serial transesophageal echocardiogram (TEE) should be performed if suspicion of valvular vegetations is high despite the initial TEE showing no vegetations.
- The mainstay treatment of NBTE is anticoagulation and addressing the underlying condition.
KEYWORDS
Endocarditis, infective endocarditis, lung cancer, marantic endocarditis
CASE DESCRIPTION
A 44-year-old woman with a history of intravenous drug use and a 30-pack-year smoking history presented to the emergency department with a 2-day history of new-onset substernal chest pain, left arm pain, and ataxia. Other pertinent history included a 2.5 cm thick-walled cavitary lesion that was located in the left lower lobe about 7 months prior to hospitalization. The patient was euthermic. Pertinent physical findings included tachycardia, decreased grip strength in her left hand, and ataxia with normal strength in her lower extremities. No murmurs were noted.
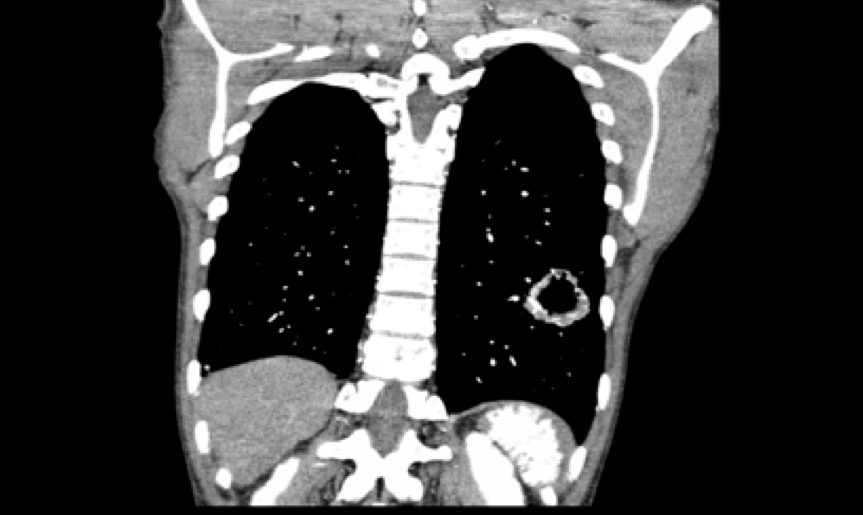
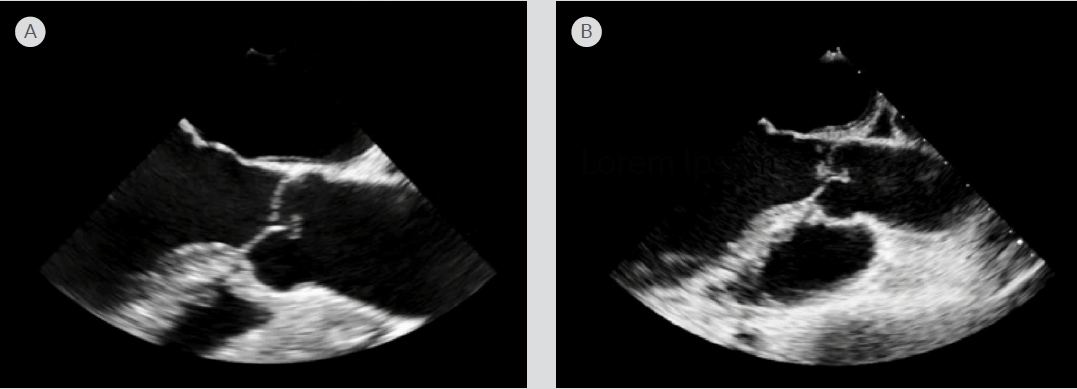
C-reactive protein was elevated at 1.8 mg/dl. Serial troponin I peaked at 2.530 ng/ml. The white blood cell count, electrolytes, erythrocyte sedimentation rate, and antinuclear antibodies were all within normal limits. Computed tomography (CT) of the head without contrast indicated multiple subacute infarctions in the left parietal and occipital lobes. CT angiography of the chest and abdomen indicated a thick-walled cavitary lesion in the left lower lobe of the lung that had grown to 4.6 cm and multiple splenic infarctions (Fig. 1). A transesophageal echocardiogram (TEE) performed on admission did not indicate any valvular lesions (Fig. 2A). Neither the set of blood cultures drawn before antimicrobial therapy nor the seven sets drawn later indicated any growth. Subsequent magnetic resonance imaging of the head performed on admission day 2 also confirmed new bilateral acute infarctions in the frontal, parietal and occipital lobes.
Figure 1. CT of the chest and abdomen. A 4.6 cm thick-walled cavitary lesion was located in the left lower lobe of the lung with multiple wedged-shaped splenic infarctions.
Figure 2. Transesophageal echocardiogram (TEE). (A) TEE in the long axis view performed on admission with no vegetations on the aortic valve. (B) Repeat TEE performed on admission day 5 indicating a new mobile vegetation on the right coronary cusp of the aortic valve, measuring 0.5 cm by 0.4 cm, even though the patient was on antimicrobial therapy and heparin.
Repeat TEE on admission day 5 indicated moderate aortic regurgitation and a mobile vegetation on the right coronary cusp of the aortic valve, measuring 0.5 cm by 0.4 cm, even though the patient was on antimicrobial therapy (Fig. 2B). On admission day 6, the patient experienced drooping of the right side of her mouth and complete loss of movement of her right hand. Repeat CT of the head indicated more infarctions in the parietal and occipital lobes. A CT-guided biopsy of the lung indicated adenocarcinoma. The patient was diagnosed with NBTE to the CNS and spleen.
The patient was started on a heparin drip but was transitioned to enoxaparin due to her new diagnosis of adenocarcinoma of the lung. Because of the high suspicion for infective endocarditis, the patient was started on vancomycin, ampicillin-sulbactam and rifampin. Due to the serial negative cultures, the patient was de-escalated 1 week later to a 3-week course of ceftriaxone and vancomycin, for a total of 4 weeks. Despite completing a full course of antimicrobial therapy, the patient continued to have multiple cerebral vascular accidents and developed profound expressive aphasia and functional deficits in her upper extremities bilaterally, resulting in the patient being unable to perform activities of daily living. Due to the patient’s poor prognosis, she was not considered to be a good candidate for aortic valve replacement. One month after being discharged, she died of pericardial effusion and pneumonia with pleural effusion.
DISCUSSION
NBTE, also referred to as marantic endocarditis, is a paraneoplastic phenomenon with sterile vegetations on cardiac valves that are composed of fibrin and platelet aggregates[1]. The exact pathogenesis of NBTE is not fully understood, but it is believed to involve endothelial damage, hypercoagulability and circulating immunocomplexes [1]. The most common malignancy associated with NBTE is adenocarcinoma of the lung and ovary [2]. Patients with NBTE can be asymptomatic until they present with valvular dysfunction, disseminated intravascular coagulation, or systemic embolization to the CNS, coronary arteries, spleen, kidneys and extremities [1].
Advances in technology have allowed clinicians to visualize vegetations from NBTE in ante-mortem patients, which is exceedingly rare as most are discovered during autopsies. Transthoracic echocardiography (TTE) is usually performed first due to its wide availability, non-invasive nature, and ability to better visualize valvular lesions on the tricuspid valve. TEE has higher sensitivity than TTE in patients with smaller lesions, morbid obesity, abscesses, new atrioventricular block, chronic obstructive lung disease, Libman–Sacks endocarditis due to systemic lupus erythematosus, and prior cardiothoracic surgeries, especially in patients with prosthetic valves [3].
Differentiating NBTE from infective endocarditis can be a diagnostic challenge. In this patient, the modified Duke criteria suggested that she had possible infective endocarditis as she met one major criterion due to an oscillating intracardiac mass on the aortic valve and a new aortic regurgitation murmur and two minor criteria due to her history of intravenous drug use and vascular phenomena from the embolic showering [4]. Laboratory findings that suggest NBTE include the lack of leucocytosis, normal C-reactive protein, negative blood culture sets, and positive antiphospholipid antibodies [1]. As with our patient, continued vascular phenomena despite adequate antimicrobial therapy suggest NBTE.
There are no current targeted therapies for shrinking the valvular vegetations. The mainstay of therapy includes anticoagulation and addressing the underlying condition, which includes malignancy, systemic lupus erythematous, and antiphospholipid syndrome [1]. Indications for valvular repair or replacement include heart failure due to severe valvular dysfunction, new heart block, large vegetations greater than 1 cm, or recurrent embolism despite optimal medical management [5].
CONCLUSION
Visualizing NBTE valvular lesions in ante-mortem patients is rare. The uniqueness of this particular NBTE case is that the aortic vegetations and aortic regurgitation were clearly seen to develop over the span of 5 days via TEE.