ABSTRACT
Background: Coronavirus disease 2019 (COVID-19) presents with a wide range of illness severity, from asymptomatic disease to severe acute respiratory distress syndrome (ARDS). Immunosuppression is considered a risk factor for severe COVID-19, but there are only few reports on disease progression in immunocompromised patients.
Case Summary: We report the case of a 50-year-old patient with acute COVID-19 pneumonia, who had iatrogenic, clinically relevant bone marrow suppression due to accidental overdose with hydroxyurea, and decreased lung capacity due to a left-sided pneumonectomy 6 months earlier. Symptomatic treatment with oxygen supplementation and pulmonary physical therapy was initiated, and hydroxyurea was discontinued. Over 14 days, the patient’s blood counts slowly recovered, and his clinical condition gradually improved, such that supplemental oxygen was no longer necessary and he could be discharged.
Discussion: A gradual increase in neutrophil and lymphocyte counts may be preferable to dampen a potentially detrimental immunological response triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Whether patients with severe COVID-19 benefit from immunosuppressive therapy should be further evaluated.
LEARNING POINTS
- Acute respiratory distress syndrome is a serious complication in COVID-19 and appears to be triggered by a proinflammatory cytokine storm.
- Immunosuppression may avoid an immune hyper-response triggered by SARS-CoV-2.
KEYWORDS
COVID-19, neutropenia, pneumectomy, clinical course
INTRODUCTION
COVID-19 presents with a wide range of illness severity, from asymptomatic disease to severe ARDS [1]. Severe COVID-19 appears to be triggered by an exaggerated immune response mainly characterized by a proinflammatory cytokine storm [2]. While pre-existing immunodeficiency is considered a risk factor for severe illness, there are few reports on disease progression in immunocompromised patients.
CASE DESCRIPTION
A 50-year old patient presented to our emergency department after referral by his general practitioner with a 1-week history of dry cough, malaise, fever and moderate dyspnoea. SARS-CoV-2 was identified by polymerase chain reaction (PCR) in a nasopharyngeal swab. The patient’s medical history included a left-sided total pneumonectomy for curative treatment of adenocarcinoma 6 months previously, essential thrombocytosis for which the patient was treated with hydroxyurea, and deep vein thrombosis, for which the patient was on medication with rivaroxaban.
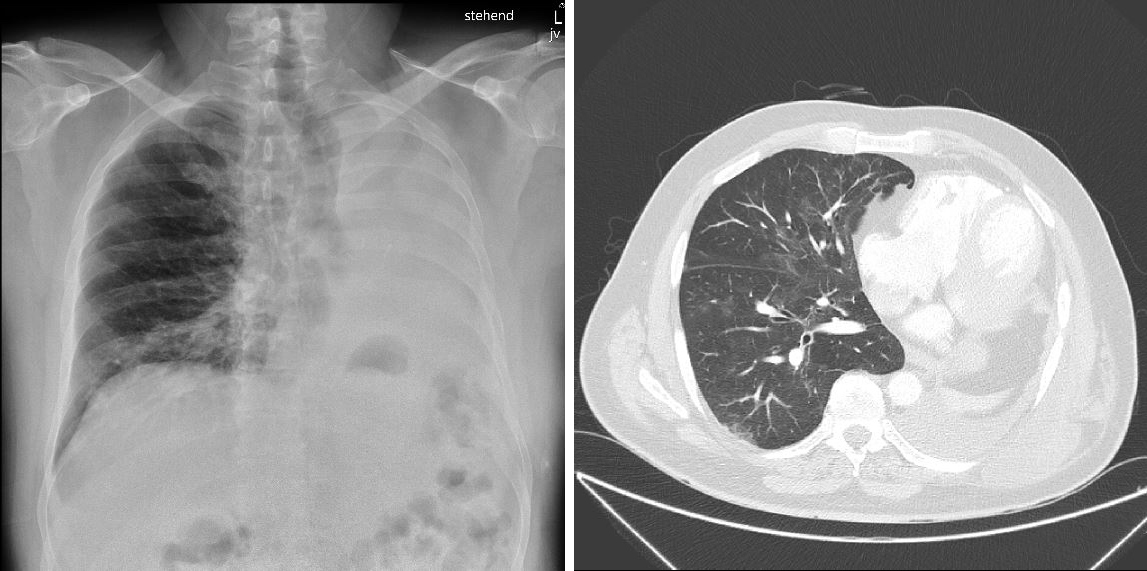
At presentation, the patient was afebrile (37.5°C), with a slightly elevated blood pressure (133/78 mmHg), normal heart rate (84 bpm) and tachypnoea (30/min) (Table 1). Peripheral oxygen saturation was 98% on ambient air. A chest x-ray showed peri-bronchiolar infiltration in the right basal part of the lung and an opacified left hemithorax with a left-sided mediastinal shift due to pneumonectomy (Fig. 1, left panel). Laboratory testing revealed bicytopenia with anaemia (haemoglobin 97 g/l) and leukopenia (1.2×109/l) with absolute neutropenia (0.3 ×109/l), lymphopenia (0.68 ×109/l) and normal thrombocytes (211 ×109/l). C-reactive protein (11.3 mg/l), lactate dehydrogenase (346 U/l) and D-dimers (657 µg/l) were also elevated (Table 1). During further evaluation, it became evident that the patient had been taking far too high a dose of hydroxyurea (up to 2.5 g/day) due to a misunderstanding concerning his treatment regime. Therefore, it was concluded that the neutropenia was iatrogenic. Symptomatic treatment with hydration and pulmonary physical therapy was initiated, and hydroxyurea was discontinued. Filgrastim (granulocyte colony-stimulating factor) was not administered as we hypothesized that that rapid neutrophil reconstitution might trigger a more severe course of COVID-19 due to a more competent immune system.
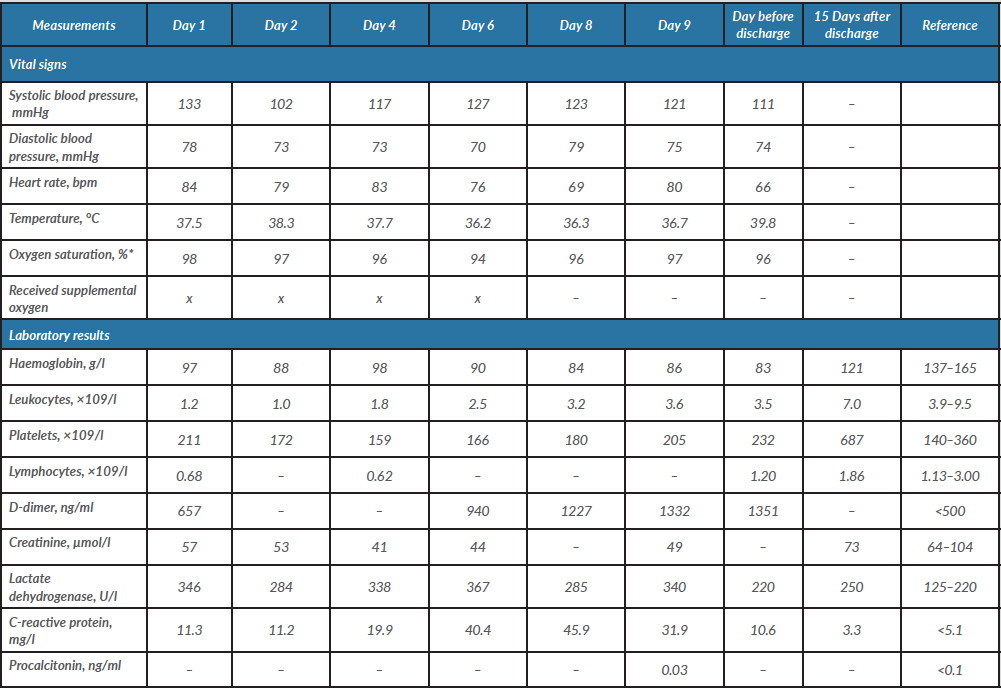
Table 1. The patient’s vital signs, laboratory measurements and radiography findings during hospitalization and at 15-day follow-up
*Measurements are without supplemental oxygen.
Over the next 10 days, the patient’s blood counts slowly recovered (leukocytes 3.5 g/l, lymphocytes 1.20 g/l, thrombocytes 232 g/l) and his clinical condition gradually improved such that supplemental oxygen could be halted on day 6. Given the possibility of a delayed immune response and the development of ARDS after the neutrophil count had recovered, the patient remained hospitalized for clinical observation and was discharged after 14 days.
At a follow-up visit 15 days after discharge, the patient had normalized haemoglobin and leukocyte counts and chest computed tomography showed residual ubiquitous mild ground-glass opacifications (Fig. 1, right panel).
Figure 1. (Left panel) Chest x-ray performed at emergency department admission showing left-sided pneumonectomy and peri-bronchiolar infiltrate. (Right panel) Chest CT scan performed 15 days after discharge showing ubiquitous ground-glass opacifications characteristic for COVID-19
DISCUSSION
This patient presented with iatrogenic, clinically relevant neutrophil suppression (severe neutropenia) due to accidental overdosing with hydroxyurea, and lymphopenia which was lower than would be expected from viral disease alone. It has been thought that SARS-CoV-2 might trigger an excessive anti-viral immune response causing massive overproduction of inflammatory cytokines and tissue damage [3]. In severe COVID-19 disease, this overwhelming immune activation, known as a cytokine storm, is a major cause of severe disease and death regardless of pathogen burden [4]. High levels of cytokines from both T-helper-1 and T-helper-2 cells have been found in patients affected by COVID-19 [3]. We therefore postulate that a gradual increase in the lymphocyte and neutrophil count instead of pharmacological stimulation may be preferable to avoid an additional immune hyper-response triggered by SARS-CoV-2.
While the patient's clinical course might have been good regardless of neutrophil or lymphocyte count, there is the possibility that the coincidental iatrogenic immunosuppression might have delayed or inhibited a potentially detrimental immunological response. In support of this hypothesis, preliminary results of the RECOVERY trial showed that administration of dexamethasone significantly reduces mortality rates in COVID-19 patients [5].
Our patient was classified as having acute COVID-19 based on pulmonary infiltration at admission necessitating oxygen support shortly thereafter. A common strategy to treat acute infection in medication-induced neutropenia includes the administration of filgrastim to stimulate neutrophil production. However, we did not administer filgrastim because we suspected rapid neutrophil reconstitution might cause immune hyper-activation. We think this report may be helpful for other clinicians making decisions regarding patients in similar settings. To date, several clinical trials are testing the efficacy and safety of monoclonal antibodies against cytokines or their receptors (such as tocilizumab) in patients with COVID-19 pneumonia.
This case report from a single patient has several limitations, notably the lack of chest computed tomography at admission to assess initial lung involvement. Further reports of COVID-19 patients with neutropenia are required, as is further research into the pathophysiology of the immune response in severe COVID-19 to guide the development of treatment strategies for immune modulation.