ABSTRACT
A 61-year-old man presented with high fever, and severe back and abdominal pain following transrectal ultrasonography (TRUS)-guided prostate biopsy. Diagnosis of spondylodiscitis and psoas abscesses was made based on MRI images of the lumbar tract of the spine. Six-monthbroad-spectrum antibiotic treatment and immobilization with a girdle overcame the disease without any relapse at the 1-year follow-up.
Spondylodiscitis after TRUS-guided prostate biopsy is a rare event, which is not yet included as a major complication of the procedure. It is probably due to thepresence of fluoroquinolone-resistant bacteria in faeces. It is, therefore, important to highlight this possibility and to stressthe use of targeted antibiotic prophylaxis after rectal flora swabbing with selected antibiotics at sufficient concentrations to be effective.
LEARNING POINTS
- Spondylodiscitis must be suspected in patients who complain of septic fever and back pain followingTRUS-guided prostate biopsy.
- In these cases, early diagnosis and rapid initiation of treatment are critical to prevent or atleast minimize this severe complication.
- Although prophylactic fluoroquinolone is the first choice in preventing acute bacterial prostatitis after TRUS-guided prostate biopsy, thepresence of fluoroquinolone-resistant E. coli in the faeces must always be taken into account. Stool cultures for the detection of fluoroquinolone-resistant E. coli might be, therefore, suggested before TRUS-guided prostate biopsy.
KEYWORDS
Spondylodiscitis, TRUS-guided prostate biopsy, antibiotic treatment
INTRODUCTION
Spondylodiscitis is a bacterial infection of the intervertebral disk and adjoining vertebrae. It is more frequently associatedwith conditions such as infections, malignant diseases or collagenosis.
Medical interventions may lead to iatrogenic spondylodiscitis either directly by inoculation or indirectly by haematogenous dissemination[1]: these include spinal procedures, urogenital and vascular interventions andintravenous catheter use. Transrectal ultrasonography (TRUS)-guided prostate biopsy is the most frequently used tool for thediagnosis of prostate cancer. It is a relatively safe method, well tolerated by patients, although minor complications and, rarely, major complications such assepsis do sometimes arise. Spondylodiscitis after TRUS-guided prostate biopsy is a rare event and few cases have been published[2].
CASE REPORT
A 61-year-old male patient suffering back pain, abdominal pain, high fever and fatiguepresented to the Department of Clinical Medicine at the University of Naples Federico II. He had undergone a TRUS-guided prostate biopsy a week beforebecause of high serum prostate-specific antigen level. Antibiotic prophylaxis (ciprofloxacin 500 mg) had been performed a day before the procedure. The histopathological features of 12 biopsy specimens showed benign prostate tissue.
High fever started with shivering one day after the prostate biopsy and back pain a fewdays later. He was treated with analgesic drugs and antibiotic (nebicine) for a week.
On physical examination, he complained of severe pain at the L2–L4 level of the spinal column and abdominal pain on palpation; neurologicalexamination only showed bilateral lumbosacral nerve root irritation since pain was elicited when the legs were raised to an angle of almost 30° (positive Lasègue’s sign bilaterally, particularly on the right). The patient still had septic fever (peak temperature 40°C).
Laboratory tests showed neutrophilic leucocytosis (white cells 12,400/ml and neutrophils 9,900/ml) and other positive signs of generalinflammation (high level of C-reactive protein (CRP), 17.8 mg/dl; normal values, 0–5 mg/dl). Serological tests for Brucella and Salmonella; purified protein derivative (PPD) test for tuberculosis; and blood and urine cultures were normal.
Chest X-ray and echocardiography were negative. Lumbosacral spine X-rays showed markedreduction in the height of the L1–L3 discs.
Abdominal ultrasound showed abscesses in the middle third of the right ileopsoas muscle (36×34×51 mm) and in the proximal third of the left ileopsoas muscle (35×20 mm) as well as hypoechogenicity of the middle lobe of prostate.
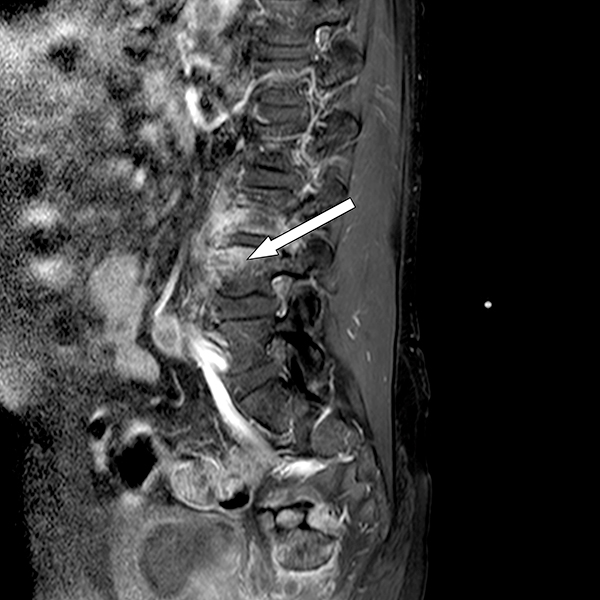
Thereafter, the patient underwent MRI, with contrast enhancement, of the lumbosacral spine.Pre- and post-contrast multiplanar images obtained by T1–T2-weighted turbo spin echo and short TI inversion recovery (STIR) sequencesshowed widespread impregnation of the upper portion of L3 and the lower portion of L2 involving the epidural anterior tissue and lateral recesses bilaterally, compressing the spinal roots as well as the psoasmuscle where abscesses were detected (Fig. 1).
Fig. 1 - Pre- and post-contrast multiplanar Images obtained by T1-T2-weighted turbo spin echo and STIR sequences showed widespread impregnation of the upper portion of L3 and the lower portion of L2 involving the epidural anterior tissue and lateral recesses bilaterally, compressing the spinal roots as well as the psoas muscle where abscesses were detected.
The patient refused to give consent for a biopsy.
Broad-spectrum antibiotic therapy, using rifampicin 600 mg i.v., ceftazidime 2 g twice a day i.v. and teicoplanin 400 mg i.v., was then started for six weeks. The patient also started to wear a metallic girdle. Fever remitted 24 hours after starting antibiotic therapy. Fifteen days later, a newabdominal ultrasound examination showed resolution of abscesses in the right and left ileopsoas. Inflammatory markers (CRP, white blood cells and neutrophils), measured bi-weekly, declined over time. Also,abdominal pain, back pain and leg pain gradually reduced.
After 6 weeks of intravenous antibiotic therapy, with negative PPD and TIBI tests, thepatient started treatment with ciprofloxacin 500 mg orally twice daily and teicoplanin 400 mg fl i.m. for six months. Liver enzymes, renal function and inflammatory markers were monitored at weeklyintervals.
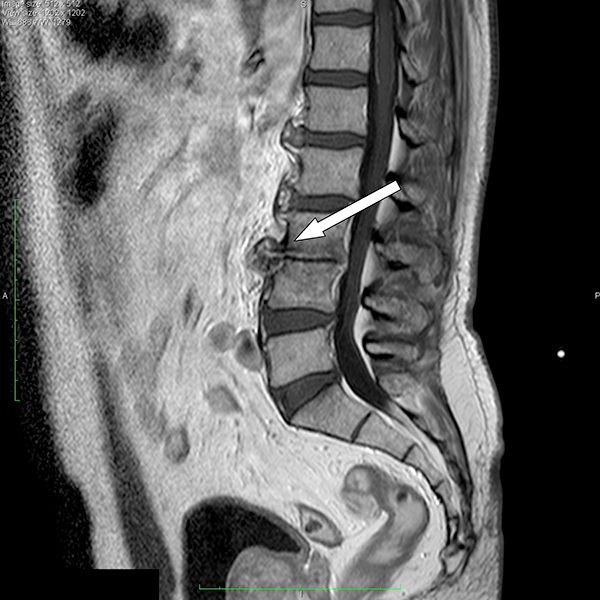
At the end of the antibiotic treatment, the patient was asymptomatic and laboratory testsshowed normal levels of white blood cells and CRP. Another MRI of the lumbar spine revealed stabilized outcome of the previous infection, showing signs of spondylosis associated with structural irregularities of the cortical bone of L3–L4. The discs included in the L1–L5 section had undergone a degenerative process (Fig. 2).
Fig. 2 - MRI of the lumbar spine performed 1 year later revealed stabilized outcome of the previous infection characterized by spondylosis associated with structural irregularities of the cortical bone of L3-L4. The discs included in the section L1-L5 had undergone a degenerative process.
DISCUSSION
TRUS-guided prostate needle biopsy is the ideal method to obtain prostate specimens forhistological analysis and is, therefore, frequently used in clinical practice[3]. It is very rarely complicated by high fever/sepsis (0.6–6.6%)1, usually caused by Escherichia coli, for inoculation of bacteria during the procedure when the needle passes through the contaminated rectum[3]. The use of antibiotic prophylaxis for TRUS-guided prostate biopsy significantly reduces the incidence of infective complicationsbut does not completely eliminate the possibility. Although fluoroquinolones are still the first choice for prophylactic antibiotic therapy, their failurein this patient might be explained by the rather frequent finding of fluoroquinolone-resistant bacteria in faeces: 13% in the first report[4] and 4% in a very recent paper[5]. Alternatively, we could suggest that treatment was not effective because it wasgiven too early, even though it was performed according to the current guidelines.
In the present report, we describe a case of spondylodiscitis, a very severe and rare complication of TRUS-guided prostate biopsy not yet includedin its list of major complications, which developed despite treating the patient with prophylactic antibiotic therapy at variance with previous cases.
On admission to the hospital, clinical presentation and laboratory analyses were typical ofan acute infection. Diagnosis was suggested by the X-ray findings of the spinal column and subsequently validated by MRI. Other possible diagnoses (neoplasticinfiltration of the bone, degenerative disk disease, intra-osseous disk herniation, primary spondylodiscitis in ankylosing spondylitis, rheumatoid arthritis) were excluded based on the clinical, laboratory and radiological findings. The clear evidenceof the disease obtained with non-invasive techniques strongly supported starting long-term treatment with antibiotics even though a biopsy, which isconsidered the gold standard for diagnosing spondylodiscitis, had not been performed.
The patient recovered with antibiotic treatment (6 weeks of intravenous antibioticsfollowed by 5 months of oral medication) and immobilization (bed rest and girdle), according to current guidelines, and no recurrence of the disease wasobserved in the follow-up period, although it is reported as possible within six months. At 1-year follow-up patient does not suffer residual disabilities,despite it is reported in 1/3 of the cases of spondylodiscitis.
The main message of this clinical case is that in patients at risk of fluoroquinolone resistance, prophylaxis of TRUS-guided prostate biopsy should be performed with carefully selected antibiotics atsufficient concentrations after rectal flora swabbing.