ABSTRACT
First described by the French surgeon Maurice Morel-Lavallee in 1853, Morel-Lavallee syndrome (MLS) is a serolymphatic effusion resulting from tangential injury to richly vascularized tissues. The imaging characteristics may be variable over time due to lesion progression and the eventual organization of a fibrous capsule. We report a case of extensive MLS in the lower leg of a 12-year-old child. We discuss the ultrasound and magnetic resonance imaging findings and describe the differential diagnoses.
LEARNING POINTS
- The diagnosis of Morel-Lavallee syndrome is based on clinical examination and imaging techniques.
- The post-traumatic context is important for diagnosis.
- Morphological aspects depend on lesion progression and the eventual organization of a fibrous capsule.
KEYWORDS
Morel-Lavallee, ultrasound, magnetic resonance imaging
INTRODUCTION
Morel-Lavallee syndrome (MLS) is a progressive post-traumatic soft tissue lesion in which subcutaneous tissue is stripped from the subcutaneous fascia, producing a cavity containing blood and liquefied fat. The radiologist must be aware of this syndrome because early identification can facilitate conservative management. When it is overlooked, MLS can be a painful mass injury that grows slowly and may be misdiagnosed as a soft tissue tumour or haematoma, particularly in a traumatic context.
CASE DESCRIPTION
A 12-year-old child was brought to the radiology department because of post-traumatic localized tumefaction of the right lower leg. He had been involved in a motor vehicle accident 2 months previously. The patient had local pain at the site of tumefaction without inflammatory signs.
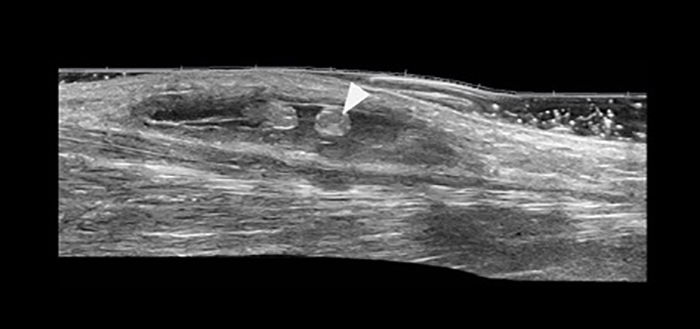
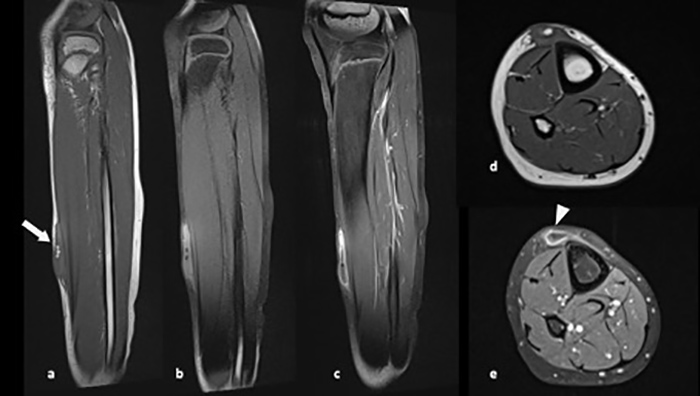
Ultrasound (US) using a high-frequency probe showed a superficial liquid collection, with well-defined margins and containing contiguous rounded formations with the same echogenicity as subcutaneous fat (Fig. 1). Magnetic resonance imaging (MRI) using the saturation pulse sequence confirmed the fatty content of the rounded formations seen on US (Fig. 2). The clinical and radiological characteristics suggested the diagnosis of MLS.
Percutaneous aspiration was performed successfully twice over 5 days and removed 75 ml and 40 ml of fluid from the collection, without complications. Compression bandages were applied for 14 days with a favourable outcome.
Figure 1. High-frequency ultrasound demonstrates an anechoic encapsulated fluid collection in the subdermal region, containing contiguous rounded formations with the same echogenicity as subcutaneous fat (arrowhead)
Figure 2. (a) Sagittal T1 and (d) axial T1 images at the level of the right lower leg demonstrate a high signal intensity elliptiform fluid collection in the superficial soft tissues (arrow), containing contiguous rounded formations, with suppressed signal on the fat-saturated T2-weighted sagittal image (b). Sagittal (c) and axial (e) T1-weighted fast spin echo (FSE) images, obtained following administration of gadolinium, demonstrate peripheral capsular enhancement (arrowhead)
DISCUSSION
Described in 1853 by Maurice Morel-Lavallee, MLS is often the result of tangential trauma to highly vascularized tissue [1]. Transaponeurotic vessels can tear when pulled. As a result, a lymphocele or haematoma containing damaged or bruised tissue develops [2]. Cases of MLS have also been reported in postoperative contexts [3].
The diagnosis of MLS is based on clinical examination and imaging techniques. Physical examination demonstrates localized skin hyperaesthesia, focal swelling and loculation. US shows a hypo-, almost anechogenic collection, located between the hypodermis and the superficial fascia, and sometimes associated with the presence of echogenic fields consisting of sheared subcutaneous fat [4]. US is also used to calculate volume and compressibility and to demonstrate the absence of flow through the collection using Doppler [5].
Acute and subacute collections have a heterogeneous hypoechoic appearance and irregular margins, in contrast to chronic collections (more than 8 months old) which are homogeneous, fusiform, and have regular morphology [6].
MRI, when available, usually shows a well-defined and fusiform collection, with signal intensity changes according to the content and chronicity of the lesion, and a surrounding pseudocapsule and hypointense ring of haemosiderin [4, 7]. There may be internal and peripheral enhancement, which is consistent with angiogenesis in the wall and the septae [8]. The protocol must include multi-planar T1 or proton density and fat-saturated T2-weighted sequences. Contrast analysis is required to rule out any solid portions suggestive of a malignant lesion [9].
Shen et al. recommended the use a simplified binary classification of acute versus chronic (if a capsule is present) to determine appropriate treatment strategies and predict outcome [10]. The differential diagnoses include subcutaneous haematoma, lymphocele and bursitis (especially in prepatellar locations). Finally, necrotic or cystic tumours can imitate MLS; however, the presence of marked enhancement, thickened walls and nodularity within the lesion are useful differentiating features [9].
CONCLUSION
MLS is a rarely reported entity. The imaging appearance is variable, and non-pathognomonic. The presence of a pseudocapsule, internal fat and subcutaneous location are helpful for diagnosis in the context of trauma. Early diagnosis facilitates conservative management.