ABSTRACT
Tracheobronchopathia osteochondroplastica (TBPO) is an uncommon benign disease, characterized by osseous or metaplastic cartilaginous nodules in the submucosa of the tracheobronchial tree. TBPO is easy to misdiagnose due to its non-specific clinical manifestation. We describe two cases of TBPO. The first patient was a 57-year-old woman with nocturnal dry cough and wheezing, in whom bronchoscopy revealed small diffuse mucosal irregularities involving the airway until the segmental bronchi. The other patient was a 69-year-old man with progressive worsening dyspnoea and productive cough presenting with severe stenosis of the trachea. Histological examination of both cases was consistent with TBPO. These cases highlight distinct forms of presentation of this rare entity.
LEARNING POINTS
- Tracheobronchopathia osteochondroplastica (TBPO) can present as a diffuse large airway disease with mild obstructive symptoms or as severe tracheal obstruction.
- Direct observation by bronchial fibroscopy of lumen protrusions sparing the posterior wall is diagnostic.
KEYWORDS
Rare diseases, pulmonology, tracheobronchopathia osteochondroplastica, bronchial fibroscopy
INTRODUCTION
Tracheobronchopathia osteochondroplastica (TBPO) is an uncommon disease affecting the lumen of the tracheobronchial tree and characterized by multiple submucosal osteocartilaginous nodules that project into the lumen of airways, sparing the posterior wall [1, 2]. It is an idiopathic non-malignant disease of the large airways, with focal or diffuse presentation. The clinical manifestations of the disease are variable, and its symptoms lack specificity. Although direct observation of the lesions by bronchoscopy remains the gold standard for TBPO recognition, other diagnostic methods including chest computed tomography (CT) and biopsy are also used [3]. To date, there is no specific treatment. By 2017, approximately 500 cases had been reported globally [4]. However, with the more frequent use of bronchoscopy, reports of this disease have increased in recent years. Here, we present two cases of TBPO and review two different facets of the disease.
CASE DESCRIPTION
Case 1
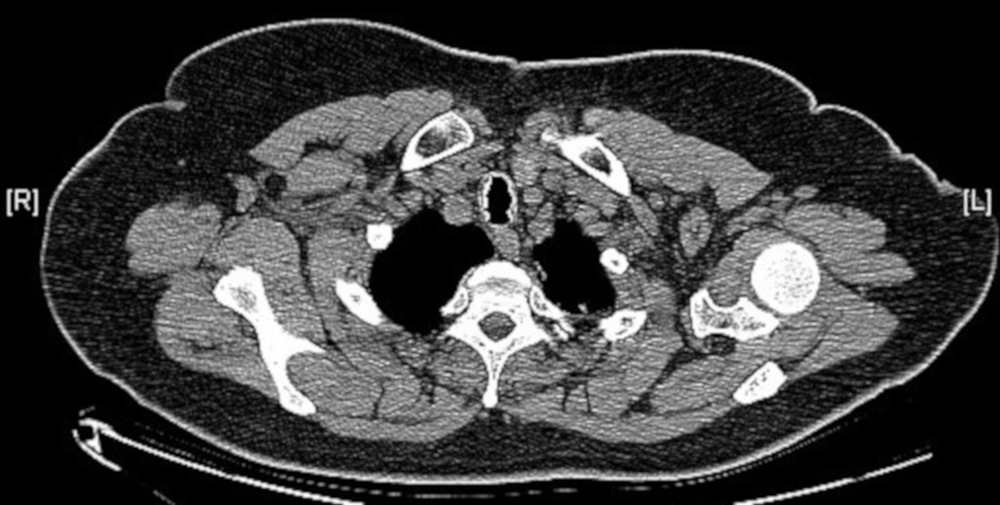
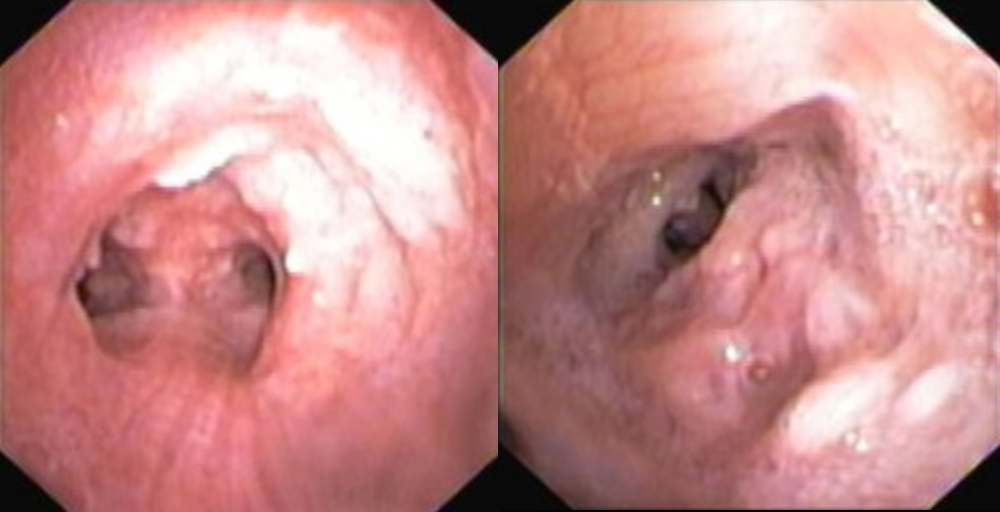
A 57-year-old woman, a non-smoker, was referred to our department from otorhinolaryngology due to irregularities of the larynx observed at laryngoscopy. She presented a 1-year history of nocturnal dry cough, and one episode of respiratory infection with haemoptysis. Physical examination was unremarkable. Spirometry revealed an obstructive pattern. Chest CT showed increased tracheal and main bronchial cartilage bone density without compromise of the lumen (Fig. 1). The patient was submitted to bronchoscopy that showed the trachea, main bronchi and left upper and middle lobes widely covered by small cobblestone nodular protrusions (Fig. 2). Histopathological examination of the biopsied tissue showed squamous metaplasia and bone formation. TBPO was considered as the cause of chronic cough, and the patient started treatment with inhaled corticosteroid with symptomatic improvement.
Figure 1. Chest CT showed increased tracheal cartilage bone density sparing the posterior wall
Figure 2. Small cobblestone nodular protrusions covering the trachea and middle lobe
Case 2
A 69-year-old man, a non-smoker, was admitted to hospital with cough and occasional white sputum for 2 years associated with shortness of breath. Physical examination was unremarkable. A spirometric test revealed severe bronchial obstruction and a
flow–volume curve suggesting fixed central airway obstruction. Chest CT showed increased tracheal cartilage bone density with significant reduction of the tracheal lumen.
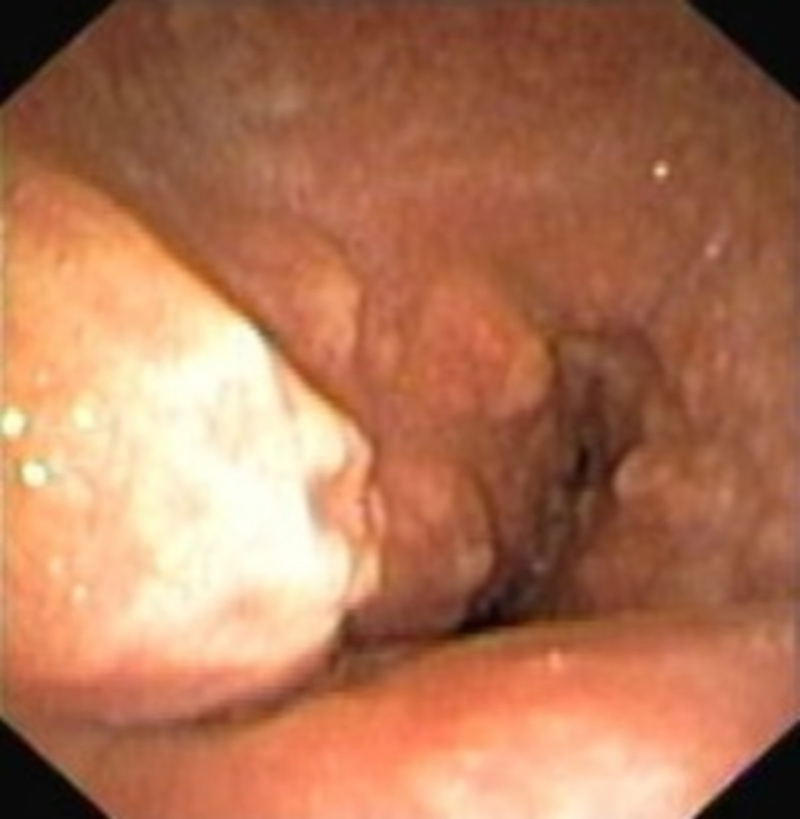
Bronchoscopy revealed rough and nodular eminences protruding into the lumen, leading to severe reduction (more than 70%) of the tracheal lumen (Fig. 3. Osseous metaplasia of the submucosa cartilage was observed histologically. The patient was submitted to therapeutic bronchoscopy with balloon dilation and argon plasma coagulation therapy, with partial destruction of the lesions. Since the patient maintained significant residual airway stenosis after debulking, and had important comorbidities, a 16/40 mm Dumon stent was placed.
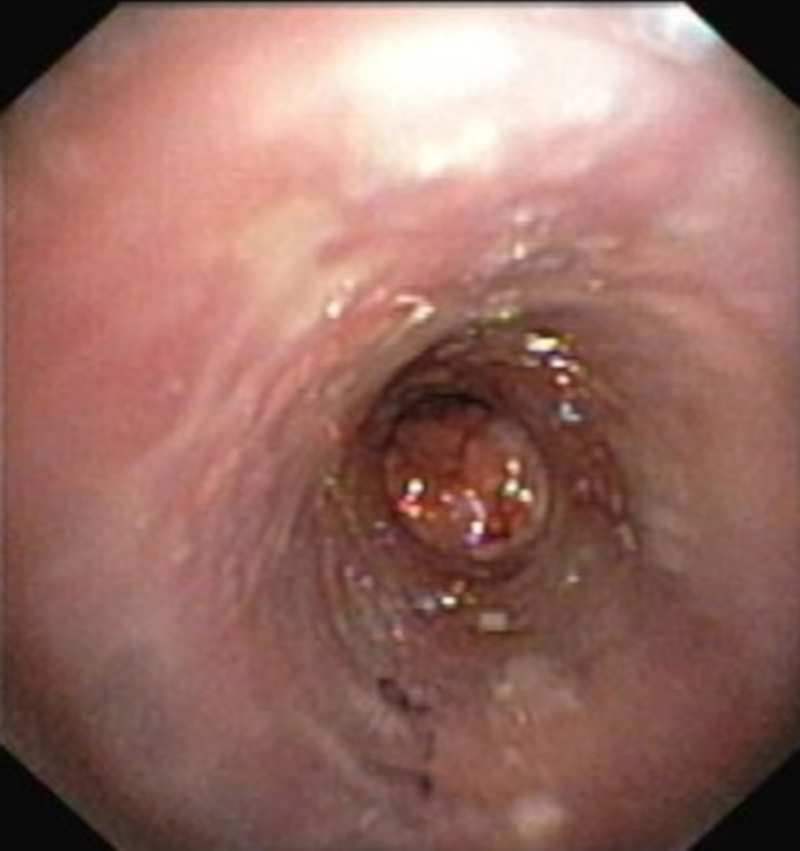
The patient is on regular follow-up with 6-monthly bronchoscopy reassessment. Five years after stent implantation the patient underwent stent replacement and argon plasma coagulation, due to recurrence of TBPO lesions and granulation tissue formation distally to the initial stent (Fig. 4).
Figure 3. Nodular eminences protruding into the lumen, leading to severe reduction (more than 70%) of the tracheal lumen
Figure 4. Recurrence of tracheobronchopathia osteochondroplastica lesions and granulation tissue formation, distal to the tracheal stent
DISCUSSION
TBPO was described macroscopically by Rokitansk in 1855 and microscopically by Wilks in 1857 [5]. Some aetiopathogenic theories have been postulated, including chronic airway inflammation, ecchondrosis and exostosis arising from cartilaginous tracheal rings and metaplasia of submucosal elastic and connective tissue. However, the aetiology remains unknown [4].
Clinical manifestations of TBPO are variable and non-specific and it presents between the fourth and seventh decades of life without any gender predominance [4]. The most common symptoms are chronic cough, sputum production, occasionally haemoptysis, dyspnoea and recurrent respiratory tract infections. Normal spirometry can be present in mild cases, while an obstructive pattern or fixed upper airway obstruction may be observed in symptomatic patients with extensive disease [6].
Chest CT may assist in demonstrating submucosal calcified nodules, but it is easy for radiologists to miss the TBPO diagnosis. Bronchoscopy is the most common and definitive diagnostic test for this condition and findings alone are often sufficient to establish the diagnosis [7]. Nodules can be seen in the lumen with bronchoscopy. Sarcoidosis, amyloidosis and papillomatosis can have a similar endoscopic appearance, but none spare the posterior wall. In some cases, however, biopsy is needed, and pathological examination of TBPO shows ossification and calcification [8].
Treatment is seldom required except in cases of severe airway obstruction when endoscopic treatment may be indicated [4].
CONCLUSIONS
These cases highlight two distinct forms of presentation. The first patient had a mild clinical presentation and extended disease. Involvement of the trachea was observed, but together with the larynx, subglottic region and segmental bronchi that are usually spared in this condition. Since the patient presented with obstruction in functional tests, bronchodilation therapy was initiated with symptomatic improvement.
The second patient had severe tracheal stenosis reflected in severe symptoms and compromise of lung function. Since airway stenosis is a serious clinical problem, bronchoscopic treatment was an important option. Although TBPO usually has a good prognosis, regular follow-up is important as the disease may progress. Moreover, the need for bronchoscopy with stent implantation may increase comorbidity due to mucus plugging with halitosis, granulation tissue formation with airway obstruction and stent migration [9].