ABSTRACT
Cholesterol embolization syndrome (CES) is an atherosclerotic complication affecting different systems with various clinical manifestations, usually triggered iatrogenically by interventional and surgical procedures or thrombolytic therapy, although spontaneous cases have been reported. The hepatobiliary system can also be affected when the showered cholesterol crystals obliterate small vessels within this system causing both ischaemic and inflammatory responses. We describe a case of a male patient who initially developed multiple lacunar cerebral infarcts 10 days post-thrombolytic therapy and percutaneous coronary intervention (PCI) due to acute myocardial infarction. Several weeks later he developed acalculous cholecystitis complicated by liver abscess and kidney injury. The consequences and latency of manifestations within different organs and the temporal relationship with well-known trigger factors raised the suspicion of CES.
LEARNING POINTS
- Cholesterol embolization syndrome (CES) is an atherosclerotic complication that usually develops after a vascular procedure/surgery or thrombolytic therapy or, rarely, spontaneously, and results in nonspecific cutaneous, renal, central nervous system and, less often, gastrointestinal manifestations that may mimic other systemic diseases.
- The delayed appearance of multi-organ manifestations from the precipitating factors may lead to difficulties in diagnosing CES.
- Complicated acalculous cholecystitis can be one of the infrequent hepatobiliary manifestations of CES.
KEYWORDS
Cholesterol crystals, acalculous cholecystitis, liver abscess
INTRODUCTION
Cholesterol embolization syndrome (CES) should be considered in the differential diagnosis when multi-organ microvascular ischaemia occurs post-vascular intervention/surgery or thrombolytic therapy, especially in patients with severe atherosclerosis and predisposing factors for cholesterol migration. The delayed appearance of multi-organ manifestations related to CES may sometimes result in difficulties in linking past vascular intervention with the diagnosis. In our case, the patient developed multi-organ manifestations at different time intervals post-percutaneous coronary intervention (PCI), which, in addition to eosinophilia, raised the suspicion of CES as the cause of different clinical scenarios post the precipitating condition.
CASE DESCRIPTION
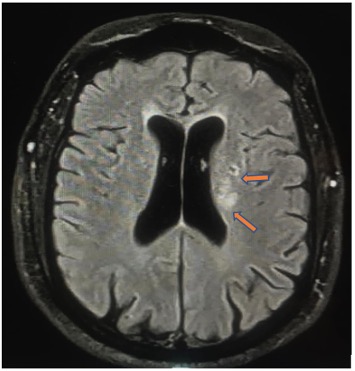
A 56-year-old male with a history of hypertension, type 2 diabetes mellitus and coronary artery disease with multiple PCIs previously was admitted with a diagnosis of inferior ST elevation myocardial infarction. He received thrombolysis therapy as per the local protocol and the next day underwent PCI where a drug-eluting stent was inserted in the distal right coronary artery. Echocardiography showed hypokinesia in the infero-posterior wall with mildly reduced systolic function of the left ventricle. He showed good recovery and was discharged on dual antiplatelet therapy after 3 days. Seven days after discharge, he presented to the emergency department (ED) with a 1-day history of confusion, mild facial asymmetry and dysarthria. No other neurological deficits were recorded. Initial computed tomography (CT) of the brain showed no evidence of bleeding and very early signs of left lacunar infarction in the basal ganglia. Magnetic resonance imaging (MRI) of the brain (Fig. 1) showed multiple left lacunar infarcts. Laboratory results were remarkable for eosinophilia and normal kidney function. With no specific treatment, the patient improved within 3 days and was discharged home in a better condition.
Figure 1. Brain MRI showing multiple left lacunar infarcts (orange arrows)
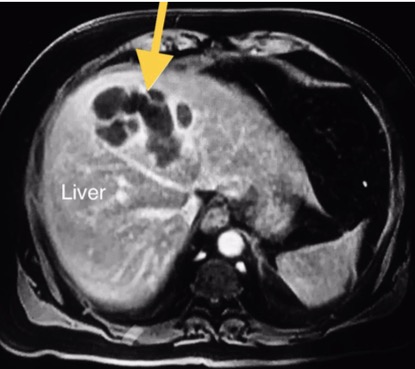
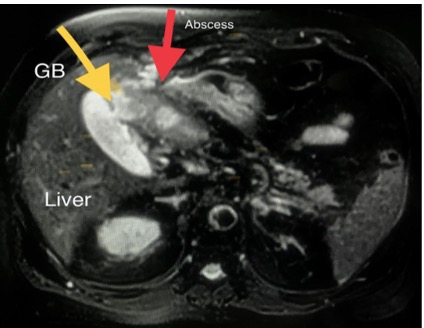
Again, he presented to the ED 4 weeks after the last discharge with right upper quadrant abdominal pain, mild fever, nausea and twice he vomited. The clinical picture was suggestive of biliary colic and abdominal ultrasound showed evidence of acalculous cholecystitis due to a thickened gallbladder (GB) wall with no gallbladder stones. Laboratory tests were remarkable for eosinophilia, normal liver function and mild renal impairment. The patient was started on antibiotics and discharged. Two weeks later, he again presented with intermittent fever, vague abdominal pain, anorexia, general fatigue and weight loss. He looked ill and dehydrated but not jaundiced. His blood pressure was 105/60 mmHg, pulse was 110 beats per minute and oxygen saturation was 98%. Examination revealed right upper quadrant tenderness. Abdominal ultrasound revealed a thickened GB wall and sludge inside with no stones, while new hypoechoic hepatic lesions were found. MRI of the abdomen showed a heterogeneous thin-walled peripheral rim-enhancing and intercommunicating cystic mass suggestive of liver abscess (Fig. 2) and a focal defect was found in the thickened anterior wall of the GB which was closely related to the liver abscess (Fig. 3).
Laboratory tests revealed leucocytosis with eosinophilia and deterioration in renal function with mildly elevated liver enzymes. Serum complement levels were within the normal range. Urinalysis gave a 3+ test result for protein and the sediment contained 1 to 4 white blood cells and red blood cells (RBCs) per high-power field, while dysmorphic RBCs were not present. Intravenous fluid therapy and antibiotics were started. The surgical team decided to carry out ultrasound-guided drainage of both the liver abscess and GB with 2 pigtail catheters and frank pus was obtained from both pigtails. The general condition of the patient gradually improved and follow-up showed a progressive decrease in the size of the liver abscess, and after 3 weeks, the patient underwent cholecystectomy.
The temporal relationship between the coronary intervention and the development of the lacunar cerebral infarcts, subacute kidney injury and the complicated acalculous cholecystitis at different time intervals, in addition to the association with eosinophilia, raised the suspicion of CES.
Figure 2. MRI of the abdomen: heterogeneous hyperintense thin-walled peripheral rim-enhancing and intercommunicating cystic mass (yellow arrow) suggestive of liver abscess
Figure 3. MRI of the abdomen: focal defect seen in the thickened anterior wall of the gallbladder (yellow arrow), which was closely related to the liver abscess (red arrow)
DISCUSSION
Cholesterol crystals (CCs) are part of atherosclerotic plaque that is usually located in the aorta and its major branches and which developed as a result of atherosclerosis. The shower of CCs from atherosclerotic plaques distally to different small vessels can result in ischaemic and inflammatory insult to various organs that determines the clinical picture of CES [1]. In the presence of adequate flow within the large vessels, microvascular ischaemia in different organs is considered the hallmark of CES in addition to inflammatory insult initiated by CCs.
It is important to differentiate between CES and a more frequently occurring condition called arterial embolization syndrome (AES), where the culprit material is a thrombus that showered from an ulcerated atherosclerotic plaque to large arteries rather than CCs, and presents a picture of acute ischaemic infarction of a distal organ in a fashion similar to arterio-arterial thromboembolism, in contrast to the slow, gradual end organ damage caused by showering of CCs to small vessels [2]. The prognosis and treatment for these 2 entities are different.
There is a significant difference between the incidence of CES diagnosed clinically and that diagnosed in autopsy studies, especially in a certain population of elderly patients who have died after aortic surgery or aortography [3], which indicates that this entity is underdiagnosed and overlooked in most cases.
The exposure of the plaque component to the systemic circulation happens due to erosion and rupture of the plaque as a result of invasive vascular procedures, the use of thrombolytic therapy and, rarely, this can occur spontaneously. 80% of iatrogenic cases are caused by angiography where coronary angiography is the most common intervention causing CES, with an incidence of 0.06–1.8% [4]. The ischaemic insult followed by inflammatory effects are considered the main pathophysiological aspects that result in endothelial injury, activation of complement and the renin-angiotensin-aldosterone systems, the release of leucocyte enzymes and subsequent end organ injury [5].
The combination of an inciting event and characteristic clinical manifestations is usually helpful in suspecting and diagnosing CES in the majority of cases, while tissue biopsy is considered the gold standard.
Skin and renal clinical manifestations are the most frequently seen with CES. Cutaneous manifestations present in 34% [1] of cases and include livedo reticularis, skin ulcers, purpura, erythematous nodules, gangrene and blue toe syndrome. Atheroembolic kidney disease resulting from CCs is characterized by progressive kidney dysfunction in a subacute fashion within several weeks, as opposed to contrast-induced acute kidney injury where kidney dysfunction usually occurs within 48–72 hours after the procedure and usually improves within 4–7 days.
The central nervous system (CNS), gastrointestinal tract and biliary system may also be affected during the showering of CCs, resulting in different clinical scenarios. The common CNS manifestations of CES relate to diffuse brain injury due to the number of emboli and the small size of the target vessels, and include confusion, memory loss and, less commonly, focal neurological symptoms and signs. CES imaging studies characterized minor ischaemic lesions and border zone infarcts [6].
Gastric and colonic ischaemia and infarction due to CES can present with abdominal pain, diarrhoea and bleeding [7], while necrotizing pancreatitis and focal hepatic cell necrosis can also be observed in the course of CES. GB involvement accounted for only 3% of postmortem diagnostic sites [8]. Acalculous cholecystitis has been reported in less than 5% of cases.
The secondary prevention of cardiovascular disease is an important aspect since CES is a manifestation of severe atherosclerosis [9]. The cessation of smoking, in addition to control of weight, blood pressure and glycaemia, is crucial. The beneficial effects of statins have been reported in CES through the lowering of low-density lipoprotein (LDL) levels and stabilization of atherosclerotic plaques with subsequent improvement in skin manifestations and renal outcome [10].
In our case, the CNS manifestations of mild confusion and facial asymmetry with brain MRI findings of multiple lacunar infarcts associated with eosinophilia occurred 10 days after thrombolytic therapy and coronary angiography, while the other organ manifestations appeared several weeks later with acalculous cholecystitis complicated by liver abscess and gradual deterioration in kidney function. The presence of the iatrogenic factors of both thrombolytic therapy and coronary angiography with subsequent multi-organ manifestations at different time intervals, and the presence of eosinophilia, should raise suspicion of CES as a cause of these different manifestations, taking into consideration the temporal relationship with precipitating factors. Fortunately, our patient responded well to initial pigtail drainage of both the liver abscess and GB with antibiotics and successfully underwent cholecystectomy later on.
CONCLUSION
Acalculous cholecystitis complicated by liver abscess can be a manifestation of CES.