ABSTRACT
Acute pancreatitis (AP) remains one of the most common causes of emergency department visits in the USA. The literature supports an association between angiotensin-converting enzyme inhibitors (ACEi), mainly at steady-state doses, and AP[1]. We present a case of recurrent AP and pseudocyst formation following multiple ACEi dose adjustments after a steady-state period lasting for over a decade. Previous reports have rarely described ACEi-induced pancreatitis and pseudocyst development. ACEi can cause significant ductal obstruction[1] and fluid retention due to its angioedema effects. Consequently, it may trigger AP complicated by pseudocyst formation. Therefore, ACEi administration must be considered in the appropriate clinical context.
LEARNING POINTS
- Although rare, ACEi is an emerging cause of drug-induced pancreatitis and often goes unrecognized.
- Multiple dose changes within a short period of time can lead to acute drug-induced pancreatitis (DIP), in addition to classic DIP caused by steady-state doses.
- ACEi-induced angioedema damages the ductal architecture and also has longer-lasting effects such as pseudocyst formation.
KEYWORDS
Acute pancreatitis, drug-induced pancreatitis, ACEi, recurrent acute pancreatitis, lisinopril
INTRODUCTION
Drug-induced pancreatitis (DIP) occurs in 0.1–2% of patients with AP and remains a diagnosis of exclusion. There are hundreds of offending agents, but ACEi are particularly important due to their numerous benefits and widespread use globally [3]. Thorough investigation of AP includes ruling out DIP among other causes. Although an association has been identified, ACEi-induced pancreatitis has rarely been described compared to reports implicating other drugs [1, 4]. The relationship between dose modification and pancreatitis development has not been fully elucidated, representing a literature gap [5]. We report a case of recurrent AP [6] and pseudocyst development in a patient whose dose was adjusted after a prolonged steady-state period. This report describes the typical presentation of DIP and discusses the pathophysiology of ACEi-induced AP.
CASE DESCRIPTION
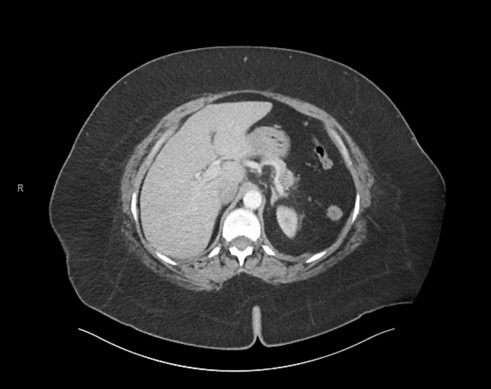
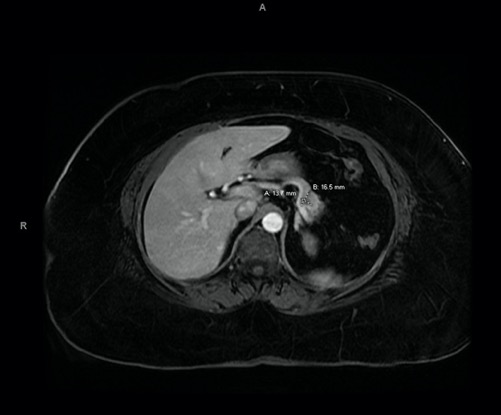
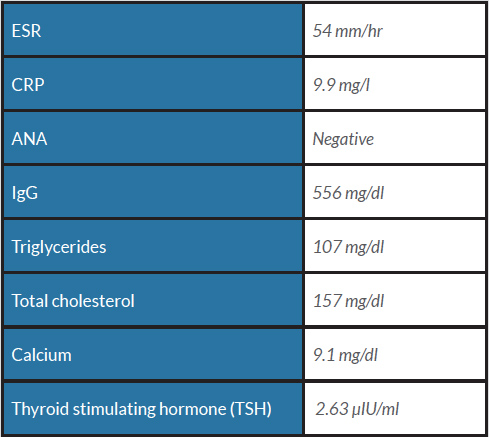
A 63-year old woman presented to our emergency department (ED) with a 7-day history of abdominal pain, nausea and anorexia (index admission). Her history included hypertension, diabetes, hyperlipidaemia, asthma and obesity. She was compliant on lisinopril 40 mg, atenolol 50 mg, hydrochlorothiazide (HCTZ) 25 mg, simvastatin 20 mg, ipratropium and insulin. She denied similar previous episodes or symptoms and did not use alcohol. She was haemodynamically stable, and physical examination only showed epigastric tenderness. Laboratory work-up revealed elevated lipase levels, consistent with acute pancreatitis. Hydration, analgesia and anti-emetics were given. Right upper quadrant ultrasound revealed no biliary pathology, while computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP) revealed a pseudocyst (Figs. 1 and 2), which had not been seen on previous abdominal imaging. The gastroenterology department was consulted and assisted in completing extensive haematological, metabolic, lipid and autoimmune profiling, which was negative.
Figure 1. Computed tomography (CT) of the abdomen, revealing a 1.5 cm hypodensity in the pancreatic body consistent with a pseudocyst
Figure 2. Magnetic resonance cholangiopancreatography (MRCP) with Omniscan revealing a 1.7 cm×1.4 cm hypoenhancement with a central cystic component
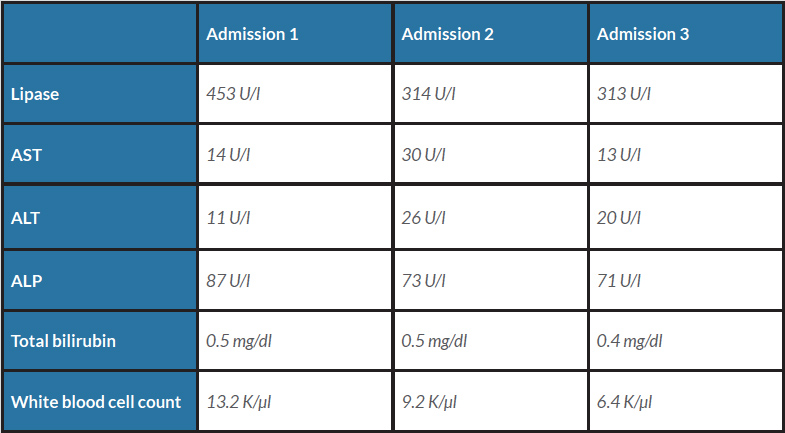
Once symptoms improved, hydrochlorothiazide and simvastatin as potential culprits were discontinued on patient discharge. Over the following weeks, the patient presented with two further episodes of pancreatitis and hypertensive urgency with residual pain, which was managed with pregabalin. At a follow-up visit, a chart review revealed previous interruption of her long-term medication regimen during an admission for asthma exacerbation and hypertensive urgency shortly before her index admission, which resulted in an increase in lisinopril from 10 mg to 20 mg and eventually 40 mg at discharge. Lisinopril was discontinued as the final potential offender. At her 2-month follow-up appointment, the patient reported no symptoms and the longest symptom-free period since episodes of AP began. Hepatobiliary trends and work-up findings are summarized in Tables 1 and 2.
DISCUSSION
The incidence of AP continues to increase and contributes an estimated $2 billion to healthcare costs [7]. Albeit responsible for a small percentage of these costs, the contribution of DIP to the overall burden can be easily reduced. Since ACEi is the first-line treatment in patients with cardiovascular pathology, ACEi-associated AP is reported in the literature in the form of case reports [1, 4] and case–control studies [5, 8]. The length of time between ACEi administration and the development of AP resulting in pancreatic angioedema ranges from hours [9, 10] to years [11]. It is postulated that bradykinin accumulation following ACEi administration increases vascular permeability, with angioedema leading to ductal obstruction [2]. This occlusion results in the entrapment of pancreatic enzymes and toxins within the pancreas which autodigest surrounding tissues [12]. Angiotensin II receptors are hypothesized to regulate pancreatic microcirculation and secretion, and the absence of receptor activation may exacerbate AP symptoms even further [13]. At the same time, ACEi can induce hypoglycaemia that has direct toxic effects on the pancreatic ducts [9]. Specific characteristics like older age and African American ethnicity [11], as in our patient, may result in higher predisposition to ACEi angioedema [14]. Our patient’s history of uncontrolled diabetes could have led to intrinsic pancreatic changes predisposing her to insult by any potential offenders [12, 14]. To support this theory, our patient’s Naranjo Adverse Drug Probability score of 6 [15] and a class III DIP designation [16] suggest an adverse drug reaction, although these scores may be under-represented as confirmatory methods such as re-challenge are unethical [9]. Our patient had been taking lisinopril for 15 years and was stable on 10 mg. Sudden adjustments in ACEi dosage, with an up-titration towards the maximum dose, can increase the burden of bradykinin, overwhelming compensatory mechanisms. These sequences of changes were seen in our patient's regimen prior to her index admission, when she was on 40 mg lisinopril at presentation.
An abdominal CT scan from years previously did not show any evidence of radiographic pancreatitis or pseudocyst formation. Since pseudocysts develop from disturbances in the pancreatic duct and extravasation of secretions [17], the initial fluctuations in lisinopril could have led to robust development of oedema and pseudocyst formation, exhibited on the index admission. The severity of ACEi-associated AP ranges from mild to severe. Most cases are mild, with a paucity of necrotic [18] or fulminant [19] cases; our patient's pseudocyst development placed her in the medium category. Although DIP is a rare entity, patients with AP of unexplained aetiology warrant careful review, and discontinuation and replacement of suspicious medications. Angiotensin receptor blockers (ARB) may be an alternative due to the lower risk of AP [20]. This case demonstrates that clinicians should be aware of the consequences of ACEi dose modification.