ABSTRACT
A patient affected by COVID-19 pneumonia may develop pulmonary hypertension (PH) and secondary right ventricular (RV) involvement, due to lung parenchymal and interstitial damage and altered pulmonary haemodynamics, even in non-advanced phases of the disease. This is a consequence of hypoxic vasoconstriction of the pulmonary circulation, the use of positive end-expiratory pressure (PEEP) in mechanical ventilation, pulmonary endothelial injury, and local inflammatory thrombotic and/or thromboembolic processes.
We report the case of a young man admitted with a diagnosis of COVID-19 pneumoniae with PH unrelated to viral infection and in whom partial anomalous pulmonary venous drainage (PAPVD) was eventually diagnosed.
LEARNING POINTS
- COVID-19 patients, even if previously well, can have pulmonary hypertension due to other causes.
- The cause of pulmonary hypertension should always be sought and not assumed, even in COVID-19 patients.
KEYWORDS
COVID-19, pulmonary hypertension, right ventricular involvement, partial anomalous pulmonary venous drainage
INTRODUCTION
Partial anomalous pulmonary venous drainage (PAPVD) is a rare congenital cardiac defect with an incidence of 0.4%–0.7% in the adult population and often associated with a sinus venosus atrial septal defect. The majority of cases are usually asymptomatic, but more rarely a patient may be symptomatic for pulmonary hypertension (PH); a prompt diagnosis can be difficult to make[1].
COVID-19 disease is a severe acute respiratory syndrome characterized by prominent pulmonary involvement that may result in PH and secondary right ventricular (RV) involvement [2]. Preliminary pathological findings demonstrate lung oedema, thickening of alveolar septa, inflammatory infiltrates and vascular congestion, which can occur in the early phase of the disease. Similarly, chest computed tomography (CT) studies showed lung abnormalities in asymptomatic infected individuals[3]. Lung parenchymal damage and altered pulmonary haemodynamics in patients with COVID-19, even in non-advanced disease stages, is a consequence of hypoxic vasoconstriction of the pulmonary circulation [4], the use of PEEP in mechanical ventilation [5], pulmonary endothelial injury [6,7] and local inflammatory thrombotic or thromboembolic processes [8]. Grosse et al. [9] reported that a key post-mortem finding in 14 cases was the presence of capillary microthrombi in 78.6% of the patients.
Therefore, in this setting, it is very difficult to make a differential diagnosis of PH, especially when CT pulmonary angiography (CTPA) findings in COVID-19 patients are not diagnostic for pulmonary embolism or difficult to realize. In addition, PH among hospitalized non-intensive care unit patients was associated with signs of more severe COVID-19 disease and with worse in-hospital clinical outcomes [2].
Here, we present the case of a young man with COVID-19 pneumonia, who was asymptomatic and had normal blood gas analysis, in whom point-of-care ultrasonography (POCUS) allowed an early diagnosis of RV involvement. The initial PH was not related to a pulmonary embolism but was eventually associated with a congenital cardiac defect.
CASE DESCRIPTION
A 22-year-old Nigerian man was admitted to a non-intensive care unit in a COVID-19 hospital with a diagnosis of COVID-19 pneumonia. He was symptomatic for fever and a nasopharyngeal swab was positive for COVID-19. He did not have a family history of cardiovascular disease, did not have a relevant medical history, and had experienced no cardiac symptoms before hospitalization.
A chest x-ray showed typical COVID-19 findings. Blood gas analysis was normal with a P/F ratio >300 and the patient did not need oxygen therapy. High-sensitive troponin T and NT-proBNP, CRP and procalcitonin were all normal. D-dimer was 2,175 ng/ml.
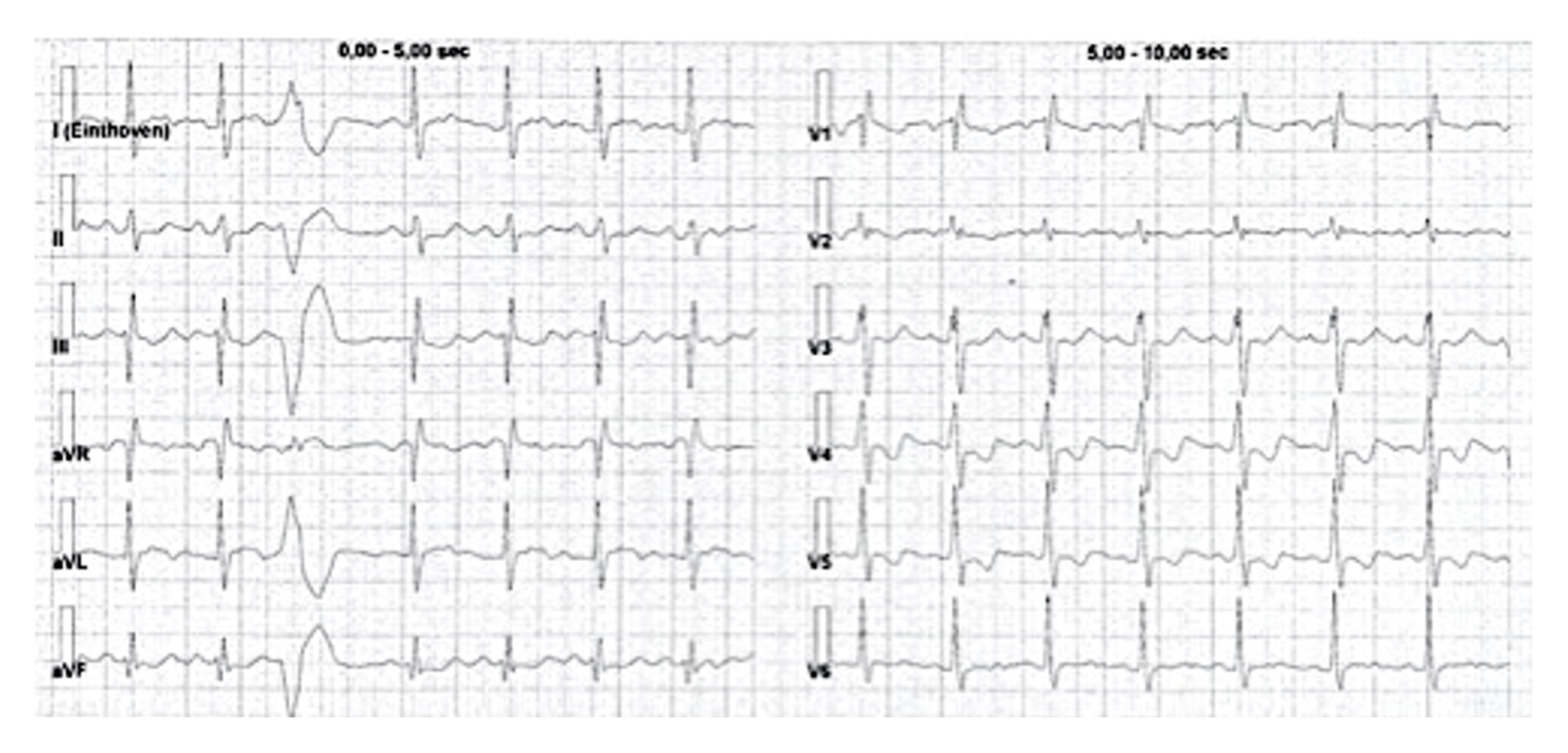
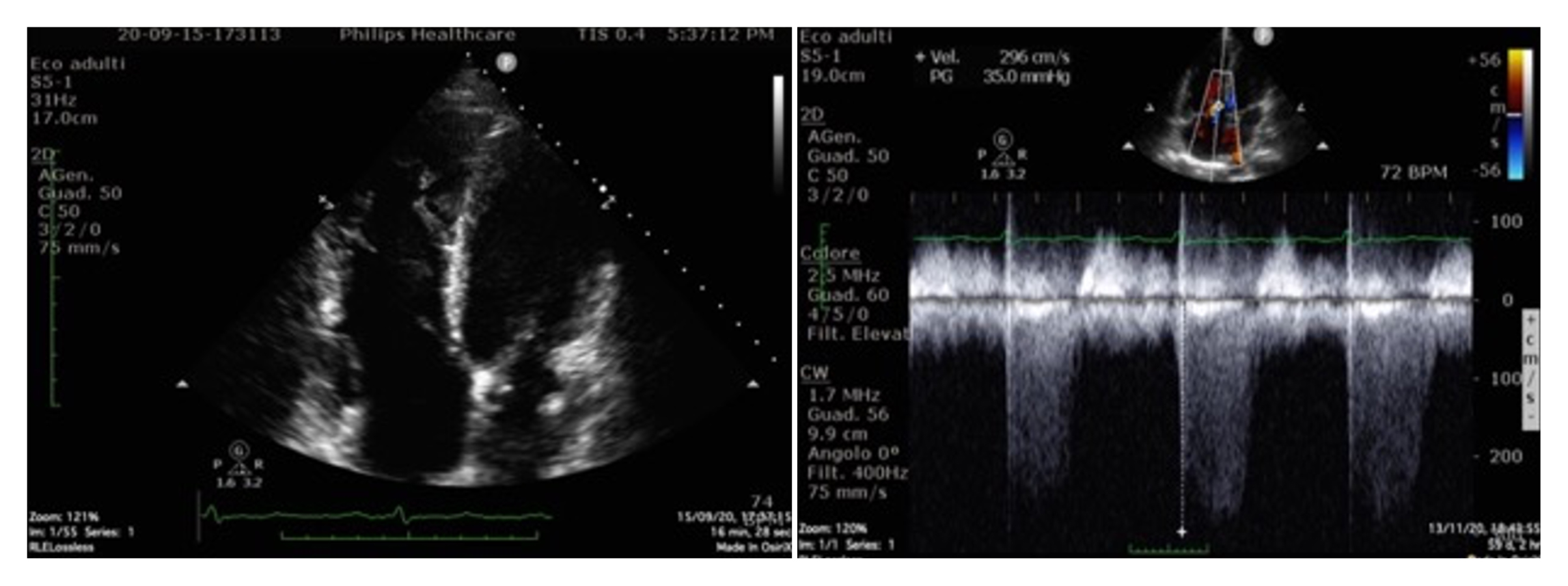
Th ECG at admission (Fig. 1) showed right bundle branch block with negative T waves in V4–V5 leads. Because of this finding, POCUS (Fig. 2) was performed and revealed the presence of severe atrium and RV enlargement, and normal left ventricular systolic and diastolic function; pulmonary artery (PA) systolic pressure was estimated at 45 mmHg
Figure 1. ECG showing right bundle branch block with negative T waves in V4–V5 leads
Figure 2. Point-of-care ultrasonography showing severe atrium and right ventricular enlargement, and normal left ventricular systolic and diastolic function; pulmonary artery systolic pressure was estimated at 45 mmHg
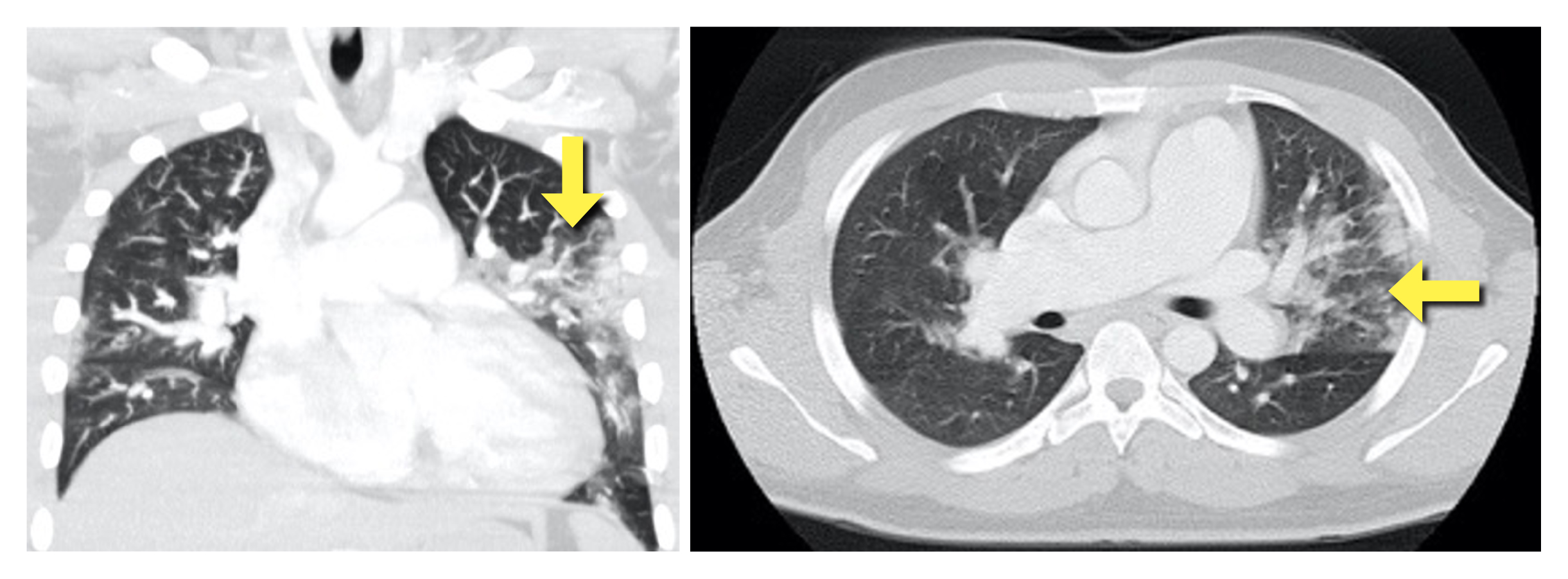
Computed tomographic angiography (Fig. 3) demonstrated multiple areas of parenchymal ground glass hyperdensity in the upper left lung lobe, in the middle lobe and in the lower lung lobes compatible with viral pneumonia (COVID-19), no endoluminal filling defects in the major pulmonary arterial branches, and enlarged right cardiac chambers with interventricular septum shift.
Figure 3. CT scan showing multiple areas of parenchymal ground glass hyperdensity (yellow arrow) in the upper left lung lobe, in the middle lobe and in the lower lung lobes compatible with viral pneumonia (COVID-19), no endoluminal filling defects in the major pulmonary arterial branches, and enlarged right cardiac chambers
When the nasopharyngeal swab became negative for COVID-19, the patient was referred to a hub centre for diagnostic invasive evaluation because of the presence of paradoxical clinical data. Right heart catheterization was performed and revealed a normal mean right atrium (RA) pressure, mean PA pressure, mean capillary wedge pressure, and a high oxygen saturation difference between the PA and the superior vena cava (SVC). The ratio of pulmonary to systemic blood flow (Qp:Qs) was 3, so a cardiac left-right shunt was documented. All coronary arteries were normal.
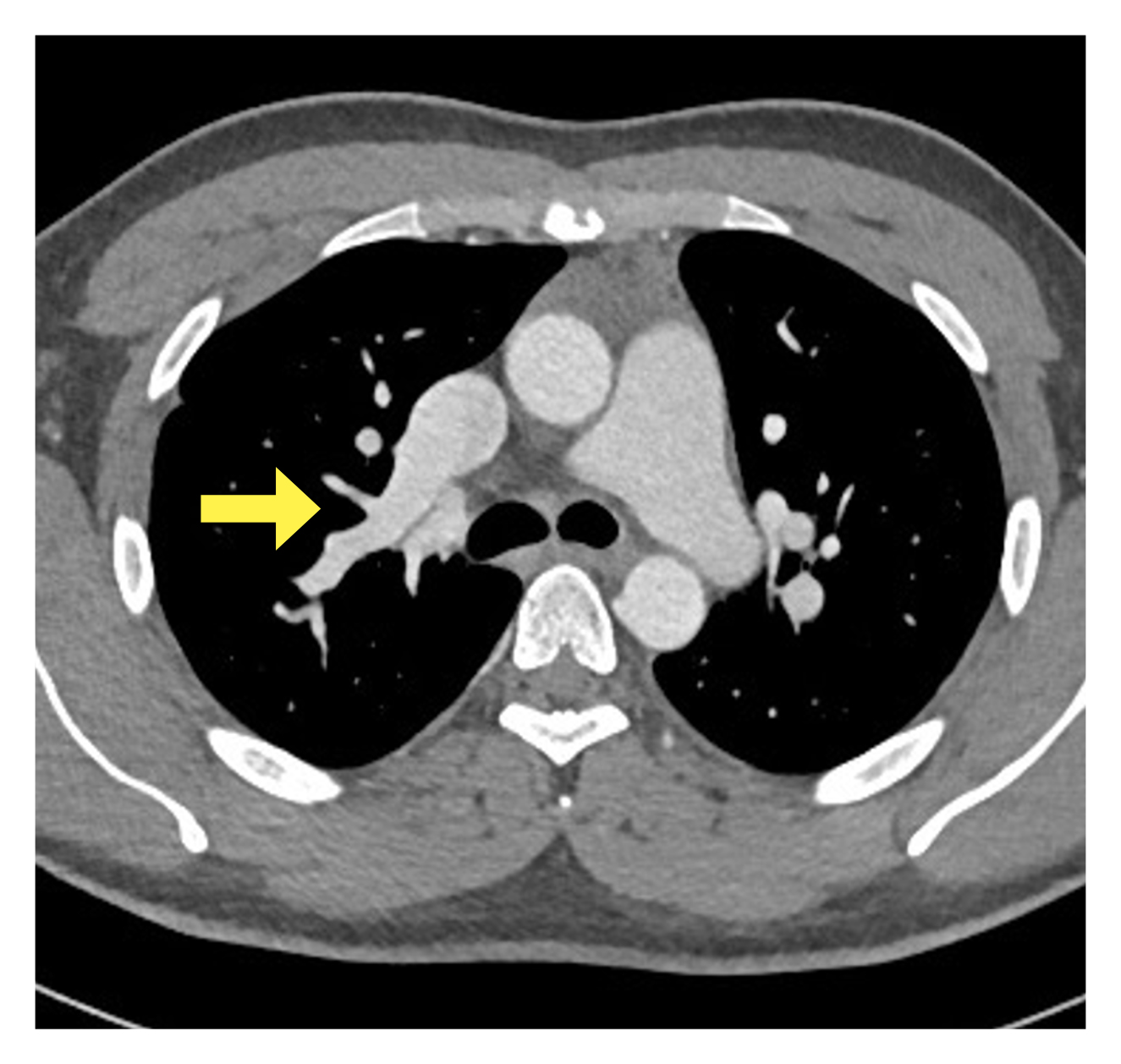
To identify the cause of the shunt, previously performed contrast enhanced CT (Fig. 4) was reviewed and revealed an enlarged right side of the heart with a large anomalous right upper pulmonary vein (RUPV) draining into the SVC. The presence of an interatrial septal defect was not clearly proven.
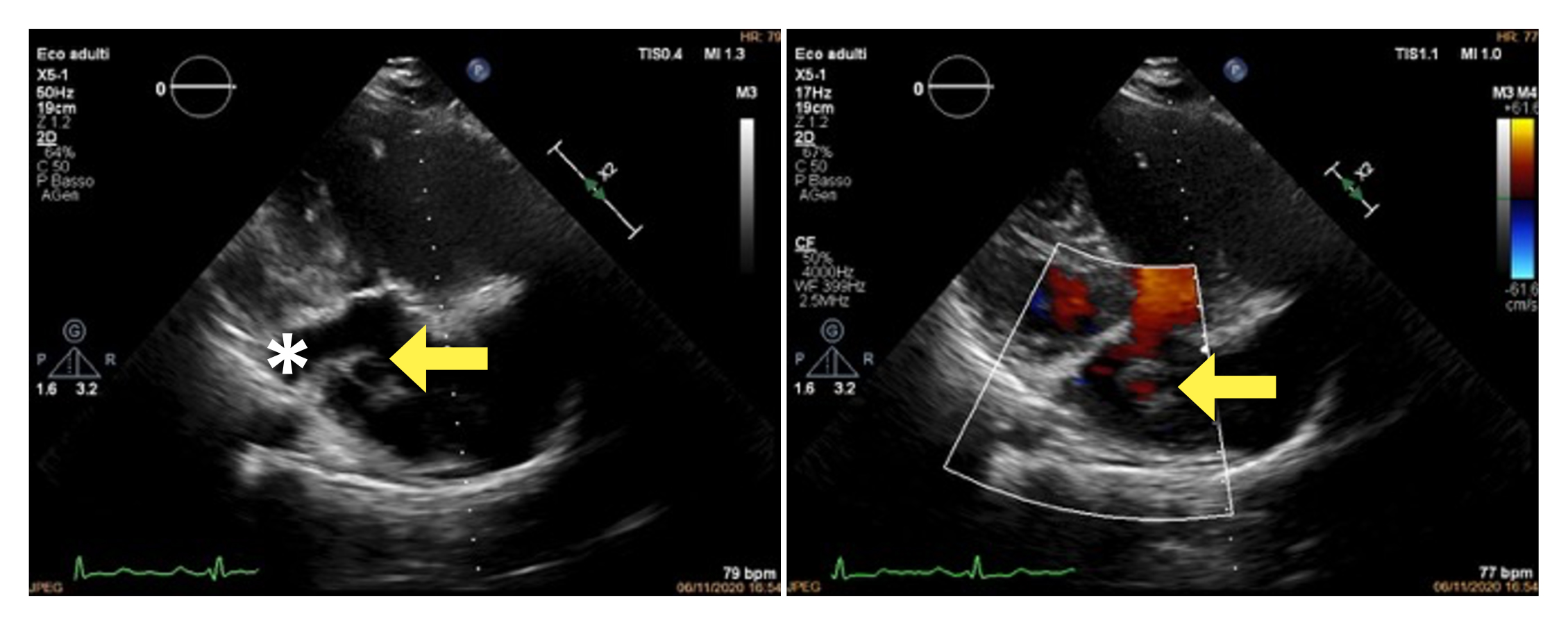
Paediatric echocardiography (Fig. 5) performed later in our laboratory showed a right pulmonary vein draining into the coronary sinus (CS) and another right pulmonary vein draining into the RA; no atrial septal defects were reported. Left pulmonary veins normally drain into the left atrium. PH was present but was lower than in the previous examination.
The patient has been referred for complete evaluation to the adult congenital heart disease centre in Padua for surgical repair.
Figure 4. CT scan showing an enlarged right side of the heart with a large anomalous right upper pulmonary vein (yellow arrow) draining into the superior vena cava
Figure 5. Paediatric modified long-axis echocardiogram showing a right pulmonary vein draining into the coronary sinus (yellow asterisk) and another right pulmonary vein draining into the right atrium (arrow)
CONCLUSION
Partial anomalous pulmonary venous connection to the CS is rare. This anomaly is even rarer in the absence of interatrial communication. Usually, the anomalous right pulmonary veins drain into the right atrium or venae cavae, while the anomalous left veins connect to the CS or left innominate vein.
This case report describes double different drainage into the CS and into the RA in a young man with initial PH, probably worsened in the setting of COVID-19 pneumoniae. The chronic RV involvement maybe explained the absence of dyspnoea pneumonia and the anomalous blood gas analysis. Moreover, when the nasopharyngeal swab became negative, the PA systolic pressure decreased, thus confirming the impact of COVID pneumonia on PH.
Finally, with this case report, the authors wish to emphasize how, in the COVID-19 era, meticulous investigation of both lungs and heart should be carried out and a diagnosis of non-viral diseases should be considered. Physicians should not focus only on COVID-19 and its consequences, despite the limits of a preliminary diagnosis in a such a difficult setting.