ABSTRACT
Multiple sclerosis (MS) can sometimes cause uncommon pseudotumoural lesions that produce atypical symptoms, such as motor epileptic seizures which are often pharmacoresistant. In these cases, accurate diagnosis is essential for correct therapy, even if unconventional. We present the case of a brain tumour in a 40-year-old relapsing-remitting MS patient who presented with pharmacoresistant seizures which eventually responded to nabiximols. After various therapeutic approaches, delta-9-tetrahydrocannabinol therapy was introduced with good results. Spasticity improved, pain decreased and we observed a reduction in the number of daily seizures. It is possible that delta-9-tetrahydrocannabinol can enhance the efficacy of anti-epilepsy therapy.
LEARNING POINTS
- The patient experienced fewer daily focal motor crises after the administration of nabiximols in the morning.
- The correct combination of symptomatic drugs can optimize a specific multiple sclerosis (MS) therapy even if the real cause of symptoms is a primary brain tumour and not MS.
- The addition of nabiximols to the therapeutic program allowed anti-epilepsy drug doses to be reduced and improved the patient's cognitive impairment.
KEYWORDS
Multiple sclerosis, delta-9-tetrahydrocannabinol, brain tumour, epileptic seizure
INTRODUCTION
Multiple sclerosis (MS) is characterized by chronic central nervous system demyelination. Patients are usually young. Rarely, magnetic resonance imaging (MRI) shows pseudotumoural lesions that produce uncommon symptoms such as epileptic seizures. In such cases, a correct and timely diagnosis is particularly important, the best combination of drugs must be chosen, and other therapeutic strategies, even unconventional ones, should be considered [1, 2]. However, combining different drugs can lead to serious side effects. To avoid all risk and improve treatment efficacy [3], we must be aware of the mechanism of action of every single drug, their interactions, and potential adverse events.
CASE DESCRIPTION
We describe the case of a 40-year-old woman who has had relapsing-remitting MS since 2015. Clinical and radiological data show low disease activity and the patient is treated with glatiramer acetate (40 mg thrice a week, subcutaneous). The patient presented after experiencing some sudden movements of the left arm and loss of conscience for a few seconds.
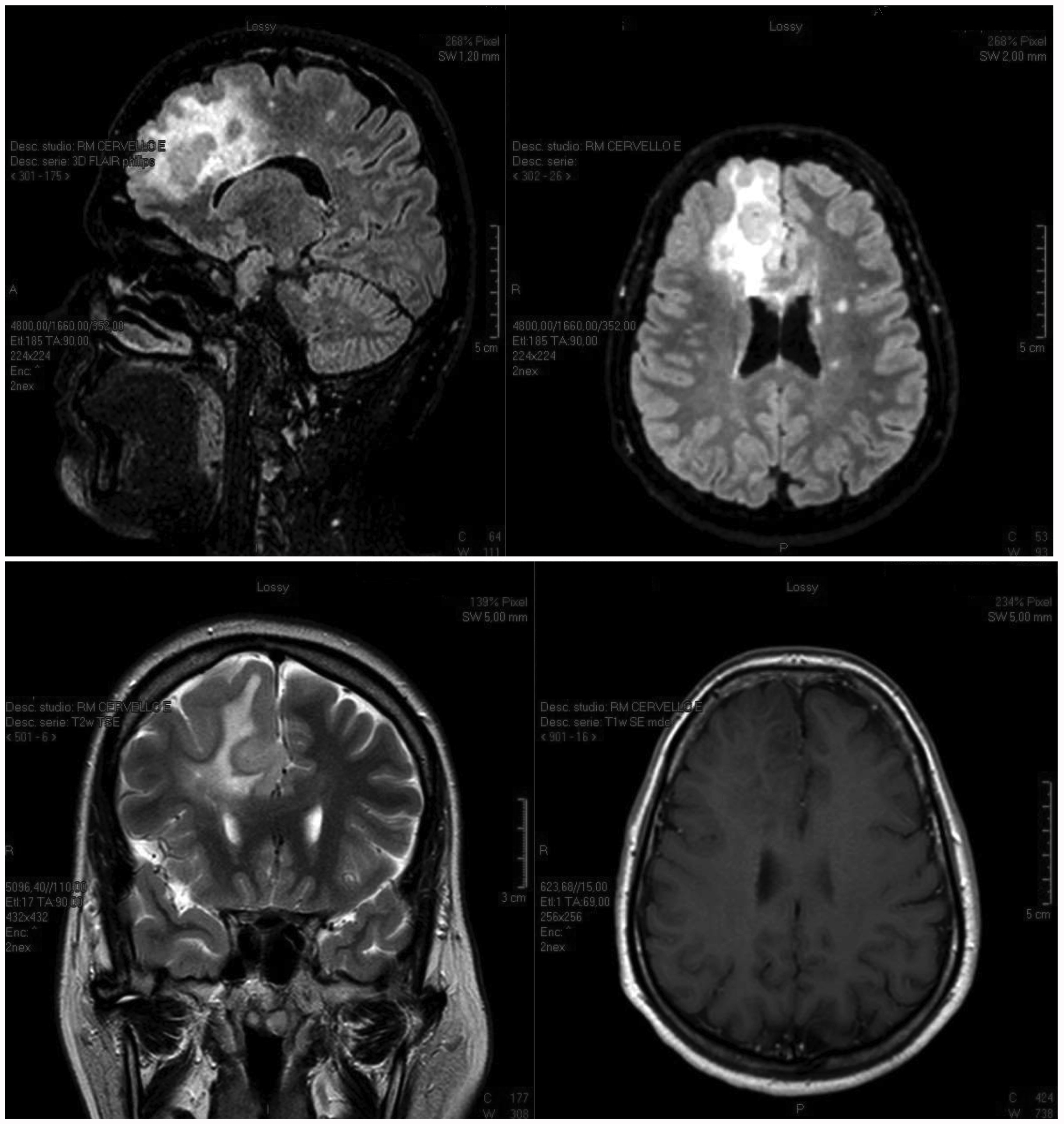
MRI revealed a very large right fronto-parietal brain lesion that originated inside the right frontal lobe and reached the homolateral corpus callosum and centrum semi vale with enhancement after gadolinium administration (Fig. 1). The electroencephalogram showed homolateral focal epileptic waves.
Figure 1. MRI shows a large pseudotumoural fronto-parietal lesion before treatment. This type of lesion can be caused by multiple sclerosis or a cerebral tumour
Sometimes, large MS lesions cause epileptic seizures that can regress after perilesional oedema is reduced with steroid therapy (such symptoms were originally considered due to MS relapse). Our patient started specific therapy with methylprednisolone 1 g intravenous (5 days) and lamotrigine 200 mg twice a day as symptomatic therapy. A second MRI showed unchanged results after steroid administration. After 3 weeks without seizures, the patient’s clinical symptoms worsened with left arm dysaesthesia and impairment of sensitivity to touch and pain. She started symptomatic therapy with pregabalin 75 mg twice a day. After 8 weeks, she presented a new epileptic seizure, so lacosamide 100 mg twice a day was also added.
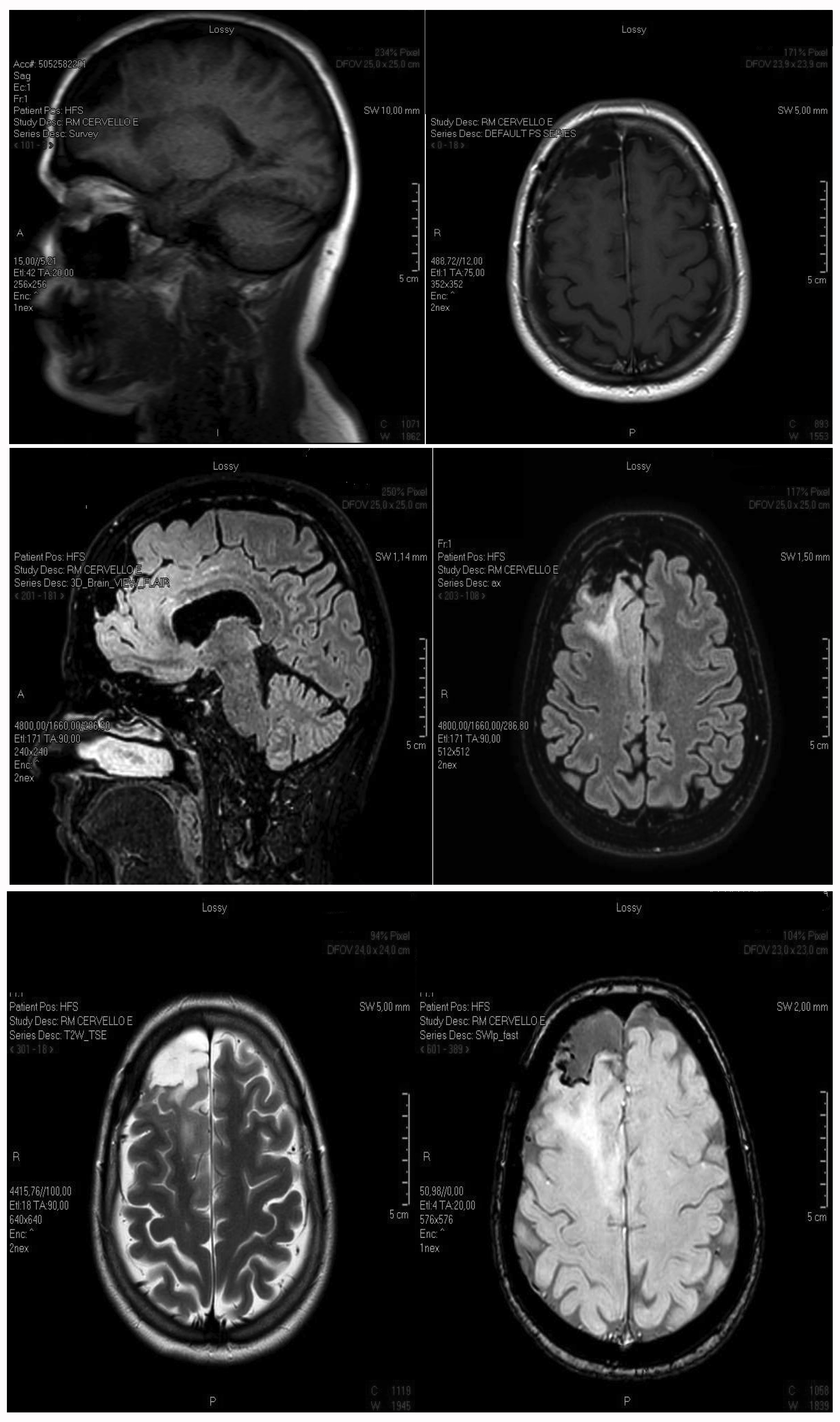
There was a possibility that the large right frontal-parietal brain lesion was caused by a primitive brain tumour. So, after neurosurgical advice, the lesion was excised with histological diagnosis of oligodendroglioma (Fig. 2.
Figure 2. MRI after surgical removal of part of a large fronto-parietal lesion, which caused pharmacoresistent epilepsy because of the affected area, extent, anatomical ratios and tissue oedema
After the operation, we observed paresis of the left arm with spasticity and pain on mobilization, impairment of left arm sensitivity to touch, frequent daily motor seizures of the left arm, and side effects due to the anti-epileptic drugs, such as cognitive mpairment, weakness and drowsiness. Steroids were necessary for 1 week (dexamethasone 4 mg, twice a day) and osmotic diuretics (Mannitol 18% 100 ml, 6 times a day).
Our patient reported a high level of diffuse pain and subjective lack of concentration, which significantly affected her work and daily activities. A test battery including the TAP (Test of Attentional Performance), TMT (Trail Making Test), SDT (Symbol Digit Test) and MFCT (Multiple Feature Cancellation Target) was normal but the patient felt slower and sleepy.
We introduced nabiximols (an endocannabinoid system modulator consisting of two active ingredients (THC, CBD) in an oromucosal spray formulation) as symptomatic therapy to decrease the spasticity of the left arm, to lower the required dose of symptomatic drugs (pregabalin 75 mg twice a day to pregabalin 75 mg once a day), and finally to reduce side effects.
After about 4 weeks of treatment with seven sprays a day, spasticity had improved, pain had decreased and the patient was able to start a rehabilitation program. She also observed a reduction in the number of daily focal motor seizures after administering nabiximols in the morning, so it was possible to reduce anti-epilepsy treatment (lamotrigine 200 mg twice a day and lacosamide 100 mg twice a day to lamotrigine 100 mg twice a day and lacosamide 100 mg once a day).
DISCUSSION
MS and primitive brain tumours can cause large cortical lesions resulting in epileptic seizures. This type of epilepsy is difficult to treat, but our data support the hypothesis that a combination of symptomatic drugs can optimize specific therapy, even if the real cause of symptoms is a brain lesion [4].
It is difficult to find a good combination of drugs to control epileptic seizures without side effects which often results in patients discontinuing therapy at follow-up [5].
Nabiximols can enhance the effect of anti-epilepsy therapy [4, 6], In fact, we successfully reduced anti-epileptic and symptomatic drug doses in our patient with an improvement in cognitive impairment [5] and decrease in seizure frequency.
In conclusion, the positive effects of combined therapy should always be investigated to improve patient welfare. Sometimes a drug combination can have an unexpected but useful interaction. It would be interesting to extend this observation to a larger number of patients to better understand our findings.