ABSTRACT
Introduction: Thymic clear cell carcinoma is the most uncommon subtype of thymic carcinoma, with 20 cases reported worldwide.
Case Description We present the case of a 61-year-old female with dyspnoea and chest pain for 2 days. Computed tomography (CT) angiography showed pulmonary thromboembolism and the existence of mediastinal and bilateral hilar lymphadenopathy, the largest infracarinal with an inferior axis of 25 mm, and also, micronodules on the left pulmonary parenchyma. The patient was admitted for aetiological assessment and underwent anticoagulant therapy. After a month, she had an ischaemic stroke, the sequelae of which proved to be fatal. The autopsy showed a mass in the superior-anterior mediastinum, with dimensions of 11×8×6 cm, corresponding to a thymus signet ring cell primary carcinoma. The immunohistochemistry study revealed that this mass was positive for AE1/AE3, CK5/6 and CK7.
Conclusion: The clinical, morphological and immunophenotypic diversity of this tumour makes its diagnosis a difficult multidisciplinary challenge, which requires a high level of clinical knowledge and accurate imaging and histological investigation.
LEARNING POINTS
- Thymic clear cell carcinoma is a very rare entity with an aggressive and nonspecific clinical behaviour.
- There are no defined diagnostic criteria, although diagnosis could be established with histologic/cytology analysis.
- There are no clear guidelines for treatment, which can include highly invasive surgery and chemotherapy or radiation therapy.
KEYWORDS
Thymic carcinoma, mediastinal tumour
INTRODUCTION
Clear cell carcinoma of the thymus (CCCT) is a rare subtype of carcinoma of the thymus, representing 3% of all cases and with only 20 cases reported in the literature [1, 2]. It is a carcinoma with significant histological heterogeneity, making its diagnosis and staging difficult [2, 3]. Thus, it generally has a poor prognosis due to its indolent and aggressive growth, leading to the involvement of vital structures and the presence of metastases in its presentation. Its diagnosis is fundamentally by excluding other primary tumours, through immunohistochemical, cytological and imaging studies [3]. Treatment is not consensual: usually, surgical resection, when possible, guarantees the curative intention; otherwise, chemotherapy and radiotherapy are alternatives [1, 4].
We highlight a case of CCCT and discuss the diagnostic dilemma before an end diagnosis was reached.
CASE DESCRIPTION
A female, 61 years old, independent, went to the urgency service with a 3-day history of dyspnoea, dizziness and sweating. Her relevant past medical history included superficial venous thrombosis (1 month prior, anticoagulated with rivaroxaban), spondyloarthropathy and dyslipidaemia. She had no family history of cancer.
Due to clinical suspicion and elevation of D-dimer levels, the patient was admitted to the hospital, having undergone CT angiography. The examination showed signs of pulmonary thromboembolism, represented by small luminal defects in both lungs, and multiple hilar and mediastinal lymphadenopathy, the most significant being infracarinal (25 mm). We started an aetiological study of the event, in a patient who had acute pulmonary embolism even though she was under oral anticoagulant therapy, raising the hypothesis of a possible oncological cause. On admission, she was started on enoxaparin at a therapeutic dose (1 mg/kg twice a day). During hospitalization, the patient underwent bronchial fibroscopy, which showed nonspecific inflammatory signs in the bronchial mucosa and an absence of macroscopic lesions, and CT of the thorax, abdomen and pelvis (TAP), with evidence of multiple mediastinal adenopathies as previously described, and pleural effusion. At that time, the patient was discharged with Doppler assessment of the lower limbs, a positron emission tomography (PET) scan and transthoracic biopsy scheduled at the hospital. Six days later, the patient was admitted to the hospital with presumable healthcare-associated pneumonia and was started on levofloxacin 750 mg daily with a good clinical response. The patient underwent the pending examinations (with the exception of the PET scan). Doppler assessment revealed no changes in the vascular permeability of the inferior limbs. The transthoracic biopsy showed an atypical histological fragment, possibly compatible with lung adenocarcinoma, with an acinar and papillary pattern. Neoplastic cells were cylindrical, with anisokaryosis, and expressed CK5/CK6, supposedly a pattern concordant with small cell carcinoma. The patient maintained a good clinical course for 1 week. At the end of this period, the patient was suddenly prostrate, with labial commissure deviation to the right, with typical signs of a stroke. A few hours later, the patient went into shock and after 2 days died, 2 months after the diagnosis.
The autopsy showed a tumour in the anterior mediastinum with dimensions of 11×8×6 cm, as well as several subpleural nodules, representing pulmonary and pleural carcinomatosis. The fatal thrombus was in the anterior cerebral artery.
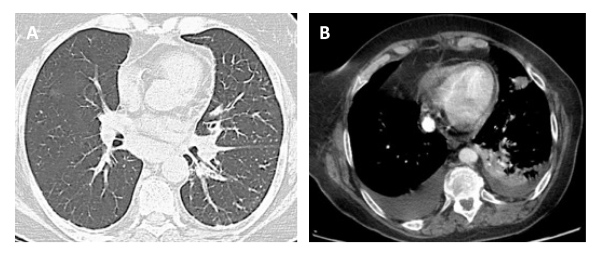
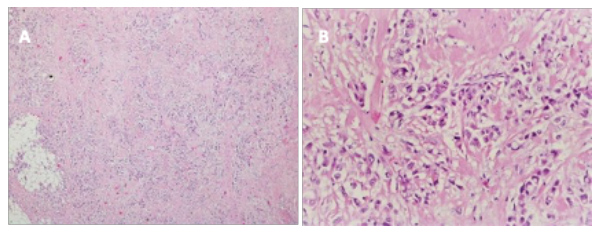
In retrospect, none of the imaging tests – CT angiography or CT TAP – observed any abnormal structure in the anterior mediastinum (Fig.1). This reinforces the idea of the high tissue heterogeneity of the carcinoma that "camouflages" it, and its rapid and aggressive growth. The case consisted of a rare neoplasia disseminated to the lung. Histological analysis revealed a signet cell carcinoma, with a glandular and acinar pattern with extracellular mucous secretion (Fig. 2).
Figure 1. CT angiography (A) and CT TAP (B) without evidence of a retrosternal mass in their multiple cuts
Figure 2. The histopathology showed clear cell thymic carcinoma with clear cytoplasm. Haematoxylin and eosin stain, x10 (A) and x20 (B)
DISCUSSION
Thymic epidermic tumours are the second most common neoplasias of the mediastinum, with a predilection for the superior-anterior mediastinum. However, thymic carcinoma is extremely rare, and within this category, the variation with clear cells is the least common[1, 5]. CCCT is characterized histologically by the presence of cells with abundant and clear cytoplasm [6]. The significant variability of cell atypia ranges from eosinophilic or lymphocytic predominance, with a well-circumscribed periphery and limited borders, to necrosis [2]. In this report, we have discussed the case of an extremely aggressive tumour, not evident in imaging studies, where possibly only PET could guide the case towards another diagnostic outcome. Researchers support the need to use this test where there is high suspicion of cancer, even if it is excluded in screening imaging tests.
The researchers thus highlight the importance of excluding primary cancer disease in the anterior mediastinum (thyroid tumours or lymphomas) or tumours that often metastasize as lung adenocarcinoma and renal carcinoma [1].
The treatment of CCCT remains unclear; however, the surgical procedure in cases of circumscribed neoplasia seems to remain the best therapeutic option. In more invasive cases, only neoadjuvant chemotherapy appeared to have some impact on survival [4, 7].