ABSTRACT
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 infection, has caused the ongoing global pandemic. Initially considered a respiratory disease, it can manifest with a wide range of complications (gastrointestinal, neurological, thromboembolic and cardiovascular) leading to multiple organ dysfunction. A range of immune complications have also been described. We report the case of a 57-year-old man with a medical history of hypertension, prediabetes and beta thalassemia minor, who was diagnosed with COVID-19 and subsequently developed fatigue and arthralgias, and whose blood work showed hyperferritinemia, elevated liver enzymes (AST/ALT/GGT), hypergammaglobulinemia, anti-smooth muscle antibody, anti-mitochondrial antibody, and anti-double-stranded DNA antibodies. The patient was diagnosed with autoimmune hepatitis–primary biliary cholangitis overlap syndrome triggered by COVID-19. To our knowledge, this is the first such case reported.
LEARNING POINTS
- COVID-19 can precipitate a wide range of immune complications; we report a case of autoimmune hepatitis–primary biliary cholangitis overlap syndrome triggered by COVID-19.
- Clinicians should be aware of this unusual manifestation of COVID-19 so that prompt and appropriate diagnostic and therapeutic interventions can be initiated if the syndrome is suspected or confirmed.
- Our case further suggests the necessity for continued and regular follow-up of patients who have recovered from COVID-19 in order to uncover the long-term effects of the novel virus.
KEYWORDS
Arthritis, autoimmune disease, COVID-19, SARS-CoV-2, autoimmune hepatitis, AIH, primary biliary cirrhosis, primary biliary cholangitis, overlap syndrome
INTRODUCTION
Acute aortic valve regurgitation is an uncommon medical emergency and is mostly caused by aortic root dissection, infectious endocarditis, sinus Valsalva aneurysm rupture, thoracic trauma or spontaneous rupture of a calcified or myxomatous aortic valve [1, 2]. We describe a 58-year-old Caucasian male weightlifter who presented with acute onset dyspnoea after finishing his intense workout program. Acute aortic valve regurgitation, due to spontaneous rupture of a bicuspid aortic valve (BAV), was diagnosed. Prompt diagnosis and surgical treatment achieved excellent long-term results.
INTRODUCTION
A novel coronavirus disease (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected millions of people worldwide since its emergence. Initially considered a respiratory tract pathogen, the virus can cause multiple organ dysfunction. Patients with COVID-19 may present with a range of immune complications including Guillain-Barré syndrome, Miller Fisher syndrome, antiphospholipid syndrome, immune thrombocytopenic purpura, systemic lupus erythematosus, Kawasaki disease, cold agglutinin disease and autoimmune haemolytic anaemia, neuromyelitis optica, NMDA-receptor encephalitis, myasthenia gravis, type I diabetes, large vessel vasculitis and thrombosis, psoriasis, subacute thyroiditis, Graves' disease, sarcoidosis and inflammatory arthritis [1]. Herein, we report the first case of autoimmune hepatitis (AIH)–primary biliary cholangitis (PBC) overlap syndrome triggered by COVID-19.
CASE DESCRIPTION
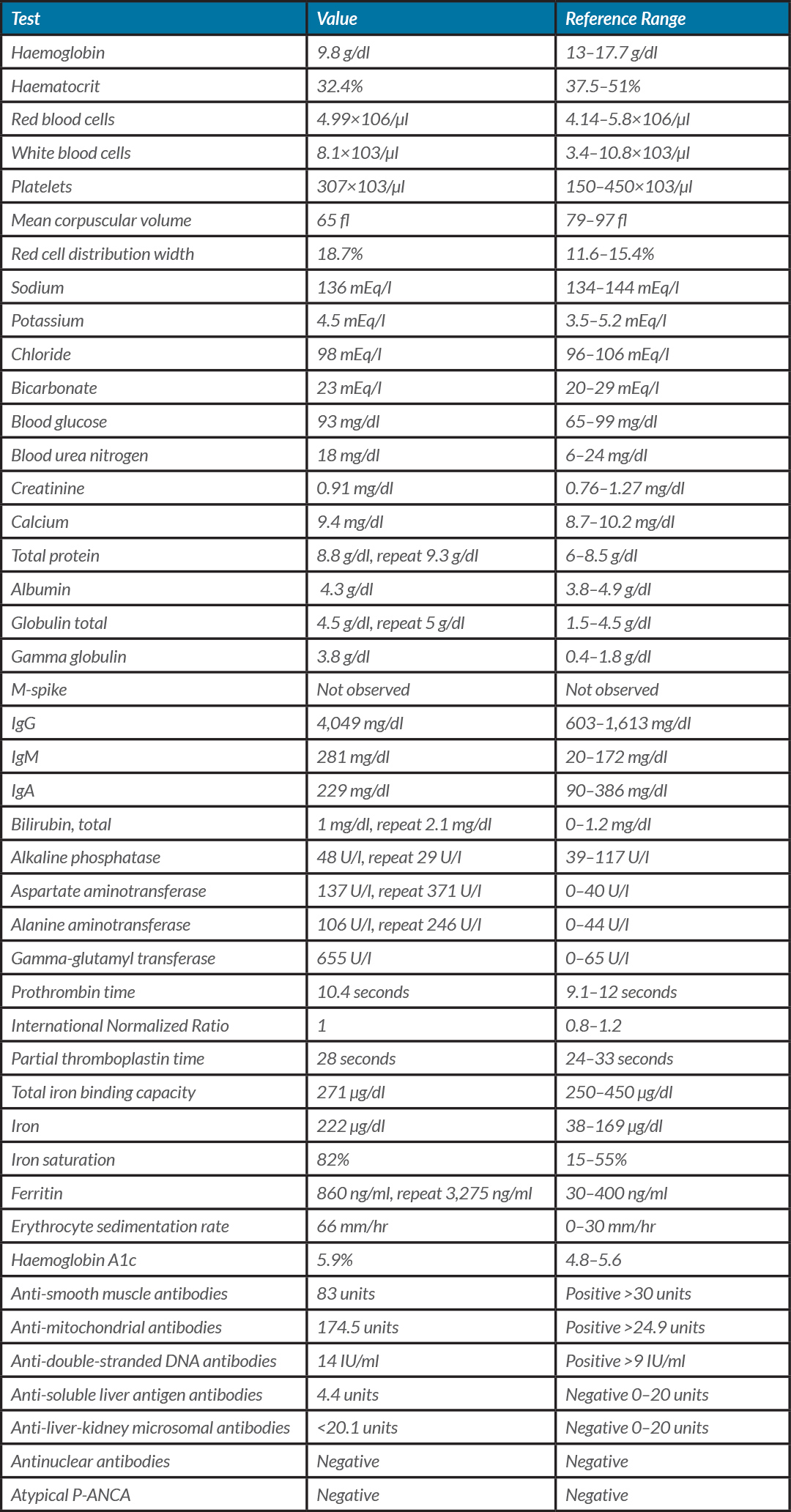
We report the case of a 57-year-old man with a medical history of hypertension, prediabetes and beta thalassemia minor, who was diagnosed with COVID-19 in April 2020. The patient developed shortness of breath and cough and was diagnosed with COVID-19 by nasopharyngeal swab RT-PCR. He was quarantined at home for 2 weeks. The patient’s respiratory symptoms resolved, but in May 2020, he started to feel fatigued and developed multiple joint pain involving the hand, wrist, knee and shoulder. The pain got progressively worse. He had not experienced nausea, vomiting, abdominal pain, itching, rash, bleeding from the nose or mouth, or blood in stools, and there was no history of alcohol or drug abuse, blood transfusions or iron supplementation. He was taking losartan, hydrochlorothiazide, fenofibrate and metformin at home. Laboratory test results are given in Table 1. The hepatitis panel including hepatitis A IgM antibodies (HA Ab-IgM), hepatitis B surface antigen (HBsAg), hepatitis B IgM core antibody (HBcAb-IgM), and hepatitis C antibodies (HC Ab) was negative, HIV was negative and body mass index was 25 kg/m2. Ultrasound of the abdomen showed a normal sized liver with mild heterogeneous parenchyma and a slightly lobulated contour. There was a 12 mm cyst in the right hepatic lobe posteriorly. There was no evidence of intra-hepatic biliary ductal dilatation and the common bile duct diameter was 6 mm. Blood flow in the main portal vein was hepatopetal. There was no evidence of gallstones. Endoscopy showed gastritis and colonoscopy showed internal haemorrhoids. Other work-up including anticentromere B antibodies, antichromatin antibodies, anti-Jo-1, anti-RNP antibodies, anti-scleroderma 70 antibodies, Smith antibodies, Sjogren anti-SS-A and Sjogren anti-SS-B was negative. Our patient was taking fenofibrate which has been associated with AIH, but he had been taking it for more than 3 years [2]. The patient was diagnosed with AIH–PBC overlap syndrome triggered by COVID-19 (given the sequence of events with COVID-19 infection followed by the onset of fatigue/arthralgias, laboratory evidence of hyperferritinemia, elevated liver enzymes (AST/ALT/GGT), hypergammaglobulinemia, anti-smooth muscle antibody, anti-mitochondrial antibody and anti-double-stranded DNA antibodies). Liver biopsy was not performed in view of the clinical presentation and serological evidence. The patient was started on ursodeoxycholic acid and continues to be followed up.
DISCUSSION
Autoimmune hepatitis is an immune-mediated, chronic, inflammatory disease of the liver is characterized by circulating autoantibodies, hypergammaglobulinemia and distinctive features on liver biopsy [3]. AIH has a wide spectrum of clinical presentation and can vary from asymptomatic disease to liver failure. PBC is a chronic, cholestatic, autoimmune disease with a variable progressive course. Patients with PBC may be asymptomatic, or they may present with signs and symptoms such as fatigue, pruritus, jaundice, cholestatic liver enzymes, antimitochondrial antibodies, and cirrhosis-related complications [4].
The term ‘overlap syndrome’ is used to describe variant forms of AIH which present with characteristics of AIH and PBC or primary sclerosing cholangitis (PSC). AIH–PBC overlap syndrome is diagnosed when two or three criteria for PBC and AIH are fulfilled. AIH is diagnosed when two out of the following three criteria are present: (a) alanine aminotransferase (ALT) levels >5×ULN, (b) serum immunoglobulin G (IgG) levels >2×ULN or a positive test for anti-smooth muscle antibodies (ASMA), and (c) liver biopsy specimen showing moderate or severe periportal or periseptal lymphocytic piecemeal necrosis. PBC is diagnosed when two out of the following three criteria are present: (a) alkaline phosphatase (AP) levels >2×ULN or γ-glutamyl transpeptidase (GGT) levels >5×ULN, (b) a positive test for antimitochondrial antibodies (AMA), and (c) liver biopsy specimen showing florid bile duct lesions [5]. Our patient met 2/3 criteria for each of AIH and PBC, although a liver biopsy was not done. Furthermore, previous reports have suggested that concomitant AMA/anti-dsDNA seropositivity can be considered the serological profile of AIH–PBC overlap syndrome as in our case [6].
The exact mechanisms leading to AIH–PBC overlap syndrome are not fully understood. In genetically predisposed individuals, viruses and drugs have been suggested as triggering agents for the autoimmune disease [2]. We report a case of AIH–PBC overlap syndrome triggered by COVID-19. Possible mechanisms include dysregulated and excessive immune responses to COVID-19 infection leading to cytokine storm and hyperferritinemia, molecular mimicry phenomena between pathogenic viruses and human proteins, and production of autoantibodies[1].
Our patient had elevated inflammatory markers (ferritin 3,275 ng/ml and ESR 66 mm/hr) suggesting an excessive immune response triggering autoimmune disease.
Clinicians should be aware of this unusual manifestation of COVID-19 so that prompt and appropriate diagnostic and therapeutic interventions can be initiated if the virus is suspected or confirmed. Our case further suggests the necessity for continued and regular follow-up of patients who have recovered from COVID-19 in order to uncover the long-term effects of the novel virus.