ABSTRACT
Despite worldwide vaccination campaigns, hepatitis B virus (HBV) infection remains a major public health problem. The natural history ranges from asymptomatic infection to severe liver injury or failure, chronic complications or reactivation episodes. The effects of HBV on the organism are immunomediated, possibly triggering extrahepatic manifestations. Since 1971, only a few cases of pleural effusion related to HBV infection have been described. We report HBV-associated pleural effusion occurring during a viral reactivation episode. Antiviral treatment directed towards pleural effusion related to HBV infection should be dictated by underlying liver disease severity and not pleural effusion severity.
LEARNING POINTS
- In the presence of pleural effusion of unknown origin, especially if with simultaneous acute hepatitis, a viral aetiology should be suspected and pursued.
- The severity of liver disease and not the pleural effusion should guide antiviral treatment.
KEYWORDS
Hepatitis B virus, viral reactivation, extrahepatic manifestations, pleural effusion
INTRODUCTION
Hepatitis B virus (HBV) infection remains a major public health problem, being the tenth leading cause of death worldwide. It is estimated that 30% of the world’s population has been exposed to HBV, with about 2 billion people infected and 240–400 million remaining chronic carriers [1, 2]. Prevalence is estimated to be 0.9% in Europe and 1.45% in Portugal [3, 4]. Its natural history ranges from acute infection that can present with no or mild hepatitis, to severe acute liver injury (ALI) or failure (ALF), to a chronic course that can lead to advanced liver disease[1]. Spontaneous or induced HBV reactivation can occur in 20–30% of patients with a quiescent chronic infection [1]. HBsAg seroclearance is the optimal outcome of HBV treatment, and can also occur spontaneously in some patients, but in either case is unusual [5].
HBV extrahepatic manifestations are immunomediated [1]. In 1971, the first case of pleural effusion associated with viral hepatitis (presumably B) was described [6]. Six years later, hepatitis antigens were identified for the first time in pleural fluid, confirming the likelihood of pleural effusion secondary to HBV infection [7]. However, although its real incidence is still unknown, this association is rarely reported nowadays. Pathogenesis is unclear, but there is probably an underlying immunological mechanism [8, 9].
We present a case of pleural effusion accompanying reactivation of HBV chronic infection in a non-cirrhotic patient, which resolved along with liver recovery. Our main purpose is to promote awareness of HBV-related pleural effusion, so the clinician is aware of this possibility in the setting of pleural effusion of undetermined aetiology in patients with an appropriate clinical context.
CASE DESCRIPTION
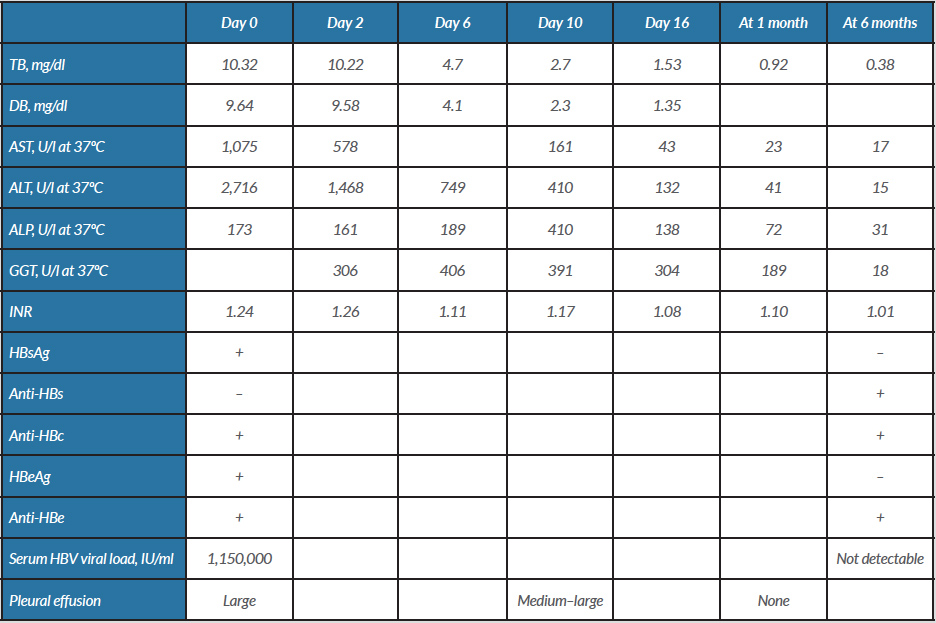
A 52-year-old man presented to the emergency department with a 5-day history of upper abdominal pain, nausea and jaundice. On physical examination, he was afebrile, haemodynamically stable, jaundiced and had a palpable and painful liver edge. There were no clinical signs of encephalopathy. The patient had a history of risky sexual contact (unprotected heterosexual intercourse) years before. There was no history of parenteral drug use, blood transfusion, HBV infection or familial aggregation. At admission, blood analysis revealed aspartate aminotransferase (AST) 1,075 U/l, alanine aminotransferase (ALT) 2,716 U/l, alkaline phosphatase (ALP) 173 U/l, total bilirubin (TB) 10.3 mg/dl, direct bilirubin (DB) 9.64 mg/dl and INR 1.24 (Table 1). Blood count, renal function and serum pancreatic enzymes were all within the normal range, and C-reactive protein was 14.9 mg/l. Abdominal ultrasound showed a normal liver and no ascites. The HBV serological profile showed positivity for HBsAg, HBeAg, anti-HBe and anti-HBc (total and IgM); anti-HBs was negative; and the HBV viral load was 1,150,000 IU/ml. Hepatitis A, C, D and E virus, cytomegalovirus and human immunodeficiency viruses 1 and 2 were negative. Immunological study for autoimmune hepatitis was negative. The patient was admitted to the Medical Intermediate Care Unit.
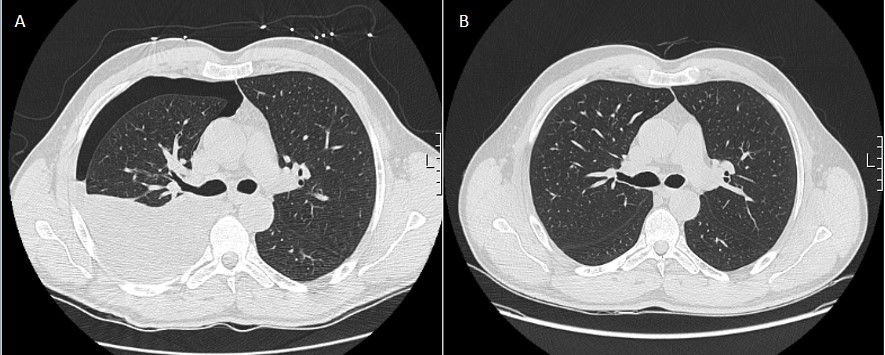
The patient also had signs of large right-sided pleural effusion at admission, without any respiratory symptoms. Computed tomography of the thorax did not show any features of parenchymal disease. Pleural liquid was classified as an exudate (according to Light’s criteria) with mononuclear predominance (1,822 polymorphonuclear and 2,957 mononuclear cells/μl); adenosine deaminase was 36 U/l (N <20 U/l) but Ziehl-Neelsen stain and mycobacteriological culture were negative, as was microbiological culture. No malignant cells were identified on anatomical pathological study and cytometry showed no evidence of a lymphoproliferative disorder. There was no clinical or serological evidence of current immunological disease. HBV DNA was identified in the pleural liquid (2,290 IU/ml). The patient developed an iatrogenic pneumothorax after diagnostic thoracentesis, which was treated conservatively.
The clinical scenario was interpreted as reactivation of a chronic HBV infection with associated pleural effusion. Best supportive care and a 5-day N-acetylcysteine protocol were initiated. The patient never showed any signs of coagulopathy or encephalopathy, and bilirubin and aminotransferase levels progressively decreased, so no antiviral therapy was started. Pleural effusion also reduced without HBV antiviral-directed therapy or diuretics (Fig. 1). The patient was discharged home 16 days later, with AST 43 U/l, ALT 132 U/l, ALP 138 U/l, TB 1.53 mg/dl, DB 1.35 mg/dl and INR 1.08. After 6 months of follow-up, the patient had a completely normal liver profile. At this time, seroconversion of HBsAg (anti-HBs >1,000 IU/l) was documented with HBeAg turning negative (Table 1), liver stiffness (by transient elastography) was 6.1 kPa and there were no clinical signs of pleural effusion.
Figure 1. Evolution of pleural effusion, at day 10 (A, with iatrogenic pneumothorax) and 1 month later (B)
Table 1. Evolution of laboratory parameters and volume of pleural effusion
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DB, direct bilirubin; GGT, gamma-glutamyl transferase; TB, total bilirubin.
DISCUSSION
We report a rare association of pleural effusion secondary to HBV reactivation, without any other obvious cause after an extensive search; malignancy, pulmonary parenchymal disease, infection other than HBV, immunological or other inflammatory conditions, and pleural effusion related to liver cirrhosis, were all ruled out. In addition, it was possible to identify HBV virus in the pleural fluid, which resolved along with HBV serum clearance, making this direct association even more plausible. Importantly, hydrothorax associated with cirrhosis was also excluded, since in addition to hydrothorax being an exudate, cirrhosis was also ruled out as the patient presented with normal liver morphology on abdominal ultrasound together with a liver stiffness value compatible with F0/F1 (no or minimal fibrosis).
The association of pleural effusion with HBV infection is rarely reported, with only, to our knowledge, 14 cases previously described; of these, only two occurred during HBV reactivation [8–10]. Unlike our case, they occurred in the setting of severe hepatitis; even so, both patients had a good outcome without targeted therapy, as did our patient [8, 9]. We propose that, in the setting of pleural effusion related to HBV infection, an accurate diagnostic aetiological work-up should be conducted with HBV viral load in pleural liquid also determined. Also, if no other cause for pleural effusion is identified, an HBV-associated aetiology must be assumed.
Extrahepatic manifestations of HBV infection are usually indications for antiviral therapy, however, given the rarity of this association, no recommendations have been published so far regarding pleural involvement. We propose that the severity of the underlying liver disease should dictate antiviral treatment in the setting of HBV-associated pleural effusion and not the severity of pleural effusion.
We also highlight two other rare events in our patient. First, spontaneous chronic HBV reactivation occurred with no identified underlying immunosuppressive condition. Second, spontaneous clearance of HBV infection after reactivation was seen.
The patient did not fulfil any recognized criteria for implementing antiviral therapy [1]. Spontaneous clearance of HBsAg is commonly seen in acute infection, but not in cases of reactivation [5]. HBsAg loss is achieved by interferon therapy in less than 11% of patients, by nucleotide analogues in less than 5%, and spontaneously in less than 2.5% [2, 11–13]. Only case reports have described spontaneous HBsAg seroconversion after reactivation flares [14, 15]. Interestingly, one of the described patients also had pleural effusion associated with HBV infection [15]. The factors associated with HBsAg seroconversion, especially after chronic HBV reactivation, remain to be determined.