ABSTRACT
Multiple sclerosis (MS) is the most common cause of non-traumatic neurological disability in young adults. It has effects at different levels: physical, emotional, psychological, cognitive and social, with a great variety of signs and symptoms. In particular, spasticity contributes to reducing the motor performance of patients with MS, causing pain, reduction in distance walked and limitations in social life. We present the case of a 39-year-old woman with MS. She was treated with delta-9-tetrahydrocannabinol/cannabidiol and the outcome was assessed with the International Classification of Functioning Disability and Health core set framework.
LEARNING POINTS
- The clinical presentation of multiple sclerosis (MS) is heterogeneous but very often lower limb spasticity leads to severe disability.
- The use of nabiximols improved spasticity control and motor performance in walking, and also had a larger effect in improving activity and participation in personal relationships.
- Appropriate assessment of MS cases, through the ICF framework, may demonstrate further effects of nabiximols on patient capacity and performance.
KEYWORDS
Multiple sclerosis, ICF, neurorehabilitation, delta-9-tetrahydrocannabinol/cannabidiol
INTRODUCTION
Multiple sclerosis (MS) is the most common cause of non-traumatic neurological disability in young adults [1]. The World Health Organization (WHO) defined health as ‘a state of complete physical, mental and social well-being and not merely absence of disease or infirmity’. The ‘bio-psycho-social’ model reflects this multidimensional concept of health, which considers the person the result of a complex and dynamic interaction of physical, psychological and environmental factors [2]. Many factors can affect the degree of disability in two individuals with the same neurological disorder. Social and economic conditions have always been an obstacle for analysing outcome measures when estimating disability in relation to the surrounding environment. A particularly useful tool is the International Classification of Functioning, Disability and Health (ICF) proposed by WHO. The ICF encompasses all aspects of human health categorized in four domains: body functions (domain b), body structures (domain s), activity and participation (domain d), and environmental and personal factors (domain e) [3]. In our centre, a screening questionnaire derived from the ICF was developed for various conditions: stroke, traumatic brain injury, amyotrophic lateral sclerosis and, recently, MS [4–7].
CASE DESCRIPTION
The ICF core set profiling study was conducted on a 39-year-old woman, who is followed in our MS outpatient service and enrolled in the Italian Multiple Sclerosis Registry [8]. The patient was diagnosed with remitting-relapsing MS 12 years previously. The neurological picture is characterized by spastic paraparesis: the Medical Research Council (MRC) scale of muscle power scored 4 on lower limbs, spasticity and pain according to the Numerical Rating Scale (NRS) scored 5, and a visual assessment scale (VAS) scored 3. The woman presented with diffuse osteotendinous hyperreflexia, slight dysmetria in the limbs with left prevalence, dysuria and nocturnal cramps in the lower limbs. She can walk autonomously and for long distances with unilateral support, presenting dysarthria and modest ideomotor slowing. The patient had normal scores on the Montreal Cognitive Assessment (MoCA) and Depression Anxiety Stress Scales (DASS-21). She lives in an isolated rural area of Umbria, in central Italy, with her husband who helps her. She does not drive, and, a few years ago, also stopped horse riding. She has been treated for spasticity for over a decade with various drugs (baclofen, dantrolene and clobazam) with discomfort from side-effects and little performance improvement. She was on immunomodulatory therapy with teriflunomide 14 mg/day. The Expanded Disability Status Scale (EDSS) score wa s estimated at 4, corresponding to the best performance, as the patient was able to walk for about 300 m without aids when she was not fatigued. However, at certain times of the day, she needed unilateral support (EDSS 6), which allowed her to walk 1 km.
At the beginning of the observation period, MRI showed a pattern of multiple demyelinating supra and infra tentorial white matter damage, in the absence of active lesions.
In February 2017, treatment with delta-9-tetrahydrocannabinol/cannabidiol (nabiximols) was proposed with 6–8 puffs spread throughout the day after 15 days’ titration. Other psychotropic drugs were suspended, and the patient was sent for evaluation by a physiatrist who delivered a rehabilitation program to improve gait. Before starting nabiximols treatment, the patient underwent evaluation using an edited version of the Brief ICF Core Set for MS. The assessment was divided into three sections evaluating three components: body functions (domain b), activity and participation (domain d), and environmental factors (domain e). More details are given in Table 1. We used this tool to investigate changes in physical symptoms and daily life functioning by comparing her condition beforetreatment with her condition after treatment with nabiximols .
After 6 weeks of treatment with nabiximols, no adverse events were observed. At 12 weeks of treatment, screening scores were satisfactory (NRS: 3, VAS: 3, MoCA and DASS-21: normal) and the patient’s autonomy had also increased. She went out more often, managed to take trips with her husband, had paraglided, and had expanded her circle of friends.
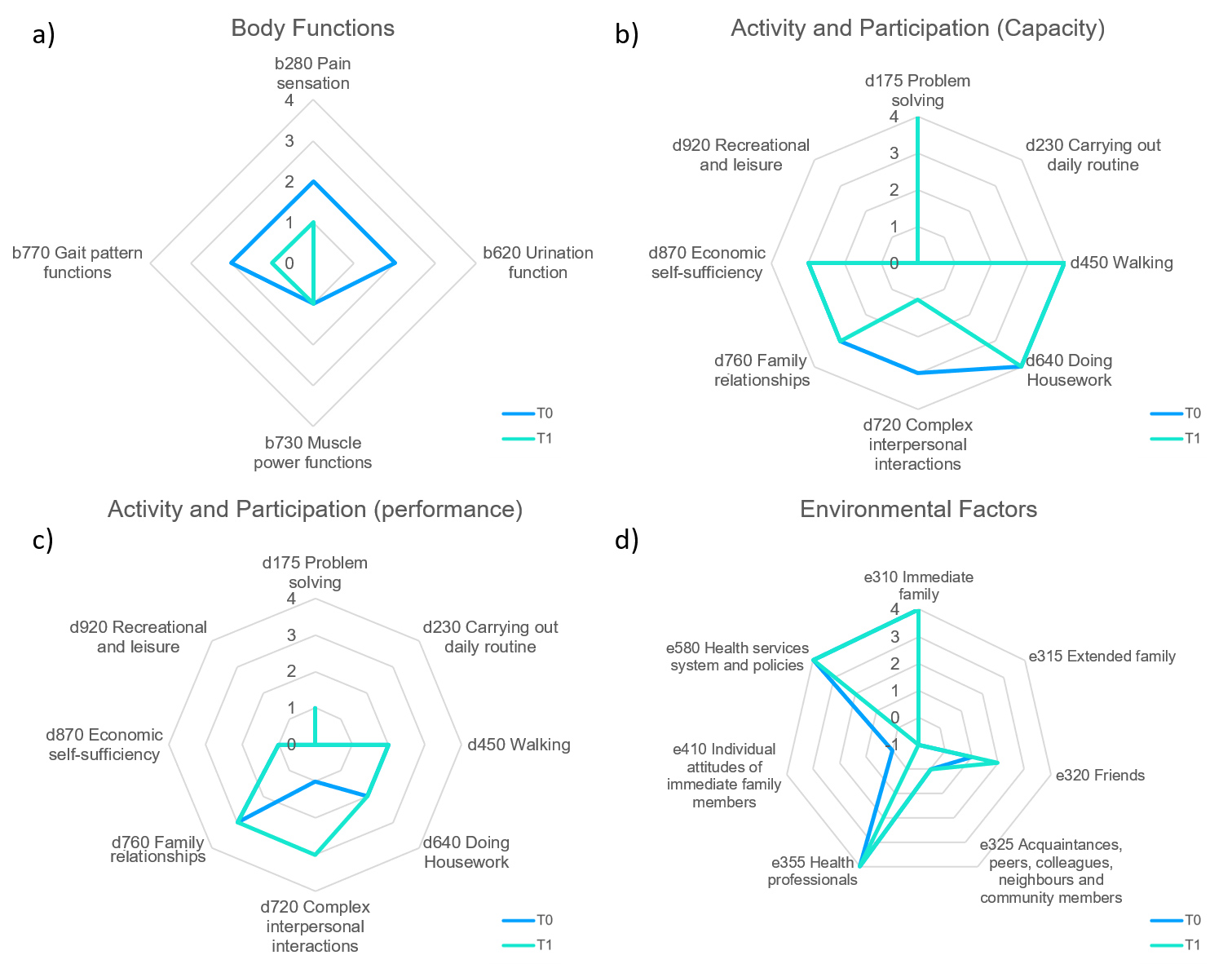
As regards body functions (domain b) assessed with the follow-up questionnaire at 12 weeks (Fig. 1A), urinary function (cumulative frequency and quality of urination), pain sensation (intensity of background and nocturnal paroxysms) and gait function (global in terms of speed and duration) had improved. muscle power remained unchanged. Regarding the activity and participation component (domain d), both capacity (what a person can do in a standardized environment, thus excluding all environmental factors) and performance (what a person actually does in her current environment with possible barriers and facilitators) showed improvement in complex interpersonal interactions (Figs. 1B and 1C). Regarding the environmental factors (domain e) component (Fig. 1D), friendships improved while individual or immediate family attitudes worsened.
Figure1. (a) Body functions: Radar Chart with the four body functions examined at initial assessment (T0) and after 12 weeks (T1): b280 Pain sensation, b620 Urinary functions, b770 Gait pattern functions,and b730 Muscle power function .Complete integrity of these functions is scored 0, which increases to 4 with maximum impairment. It is also possible to classify not specified and not applicable (xxx.8 not specified, xxx.9 not applicable) with independent codes.
(b) Activity and participation (capacity): Radar chart of the capacity inherent in the eight activities examined at initial assessment (T0) and after 12 weeks (T1): d175 Problem solving, d230 Carrying out daily routine, d450 Walking , d640 Doing housework, d720 Complex interpersonal interactions , d760 Family relationships, d870 Economic self-sufficiency, and d920 Recreational and Leisure. Complete integrity of these activities is given a score of 0, which to 4 with maximum impairment. It is also possible to classify the not specified and not applicable (xxx.8 not specified,xxx.9 not applicable) with independent codes.
(c) Activity and participation (performance): Radar chart of the performance inherent in the eight examined at initial assessment (T0) and after 12 weeks (T1): d175 Problem solving, d230 Carrying out daily routine, d450 WalkinG, d640 Doing Housework, d720Complex interpersonal interactions, d760 Family relationships,d870 Economic self-sufficiency, and d920 Recreational and Leisure. Complete integrity of these activities is given a score of 0, which increases to 4 with maximum impairment. It is also possible to classify the not specified and not applicable (xxx.8 not specified, xxx.9 not applicable) with independent codes.
(d) Environmental factors: Radar chart of the seven environmental factors examined at initial assessment (T0) and after 12 weeks (T1): e310 Immediate family, e315 Extended family, e320 Friends, e325 Acquaintances, peers, colleagues, neighbours and community members, e355 Health professionals , e410 Individual attitudes of immediate family members, and e580 Health services system and policies. Integrity of these activities is given a score of 0 , which increases to 4 with maximum impairment.
DISCUSSION
Spasticity control in MS may improve standardized outcome measures (e.g., walking speed). The rehabilitation treatment was delivered to our patient in an outpatient setting and limited to a few sessions to improve walking (maximum of 10 sessions twice a year ). It was not possible to provide treatment in an intensive setting because the patient lived in a rural area of central Umbria with poor rehabilitation services. For these reasons, the aim of rehabilitation was to maintain residual motor quotas and autonomous walking for medium distances.
Our patient had an emotional disorder with mild emotional lability (not detectable on normal rating scales). Structured evaluation of all cognitive functions was not carried out , and only the MoCA and DASS-21 scales were used. Carotenuto et al. found that higher physical and cognitive disability predicted nabiximols treatment discontinuation over 2 years in MS patients with spasticity [9]. For this reason, it would be useful to expand assessment of cognitive functions to evaluate longer treatment with nabiximols.
A broader assessment of capacity and performance may provide other benefits in daily life, activities and participation. An interview following administration of the ICF profile may offer a standardized framework for analysing overall functioning. Here, we applied the ICF profile to an MS patient, and determined the changes that took place after nabiximols treatment in body functions, activity and participation, and environmental factors. Concerning body functions (domain b), the patient reported an improvement in pain sensation (from medium to mild impairment), in urinary function (from medium to no impairment) and in gait function (from medium to mild impairment). Regarding activity and participation (domain d), she noticed an improvement in complex interpersonal interactions (from medium to mild impairment), both in capacity and in performance. Finally, with regard to environmental factors, friendships became a major facilitator, while individual or immediate family attitudes became a slight barrier. Nabiximols treatment was effective in improving some body functions and the ability to establish complex interpersonal relationships.