ABSTRACT
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus responsible for the current global pandemic, coronavirus disease 2019 (COVID-19). COVID-19 usually presents with respiratory symptoms but can affect multiple organ systems. A wide spectrum of complications can occur depending upon the comorbidities of patients. There is limited literature available regarding the presentation and outcome of COVID-19 in chronic lymphocytic leukaemia (CLL) patients. We report 2 cases of COVID-19-induced hyperleucocytosis (WBC count >100,000/μl) in CLL patients.
LEARNING POINTS
- Lymphopenia has been associated with severe disease and is a poor prognostic factor in COVID-19 infected patients; however, our cases show COVID-19-induced hyperleucocytosis (WBC count >100,000/μl)/lymphocytosis in CLL patients.
- Prior reports suggest that ibrutinib may have a protective effect against COVID-19 by decreasing inflammation and preventing progression to ARDS.
KEYWORDS
COVID-19, SARS-CoV-2, CLL, chronic lymphocytic leukaemia, lymphocytosis, leucocytosis, lymphopenia
INTRODUCTION
The pandemic caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), known as coronavirus disease 2019 (COVID-19), has contributed to a global health crisis and a magnitude of consequences. The virus has affected more than 93 million people and claimed the lives of more than 2 million individuals globally, according to the World Health Organization [1]. Risk factors associated with a poor outcome include advanced age, hypertension, diabetes, coronary artery disease and chronic kidney disease [2]. The clinical course of COVID-19 can range from mild respiratory symptoms to severe extensive lung damage and the development of acute respiratory distress syndrome (ARDS) along with multi-organ failure [3].
A nationwide analysis by Liang et al. proposed that cancer patients may be at increased risk of developing COVID-19 and those with COVID-19 had a poor outcome compared to individuals without cancer [4]. We report a case series of 2 patients with chronic lymphocytic leukaemia (CLL) who were diagnosed with COVID-19 and developed hyperleucocytosis, as well as a review of the literature on the outcome and clinical course of COVID-19 amongst patients with CLL.
CASE DESCRIPTION 1
The patient was an 81-year-old female with a past medical history of CLL (diagnosed in 2010), dyslipidaemia, hypothyroidism and hypertension who presented to the emergency room from a nursing home facility with a 1-week history of increasing fatigue, weakness, loss of appetite, dyspnoea on exertion and inability to perform daily activities. She denied any fever, nausea, vomiting or changes in urinary or bowel habits. The patient was diagnosed with Rai stage 0 CLL in 2010 with a baseline white blood cell (WBC) count of 60–70 K and was under observation. A nasopharyngeal swab tested positive for SARS-CoV-2 using the real-time reverse transcription-polymerase chain reaction (rRT-PCR) assay.
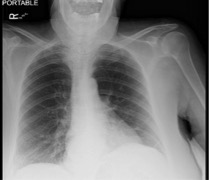
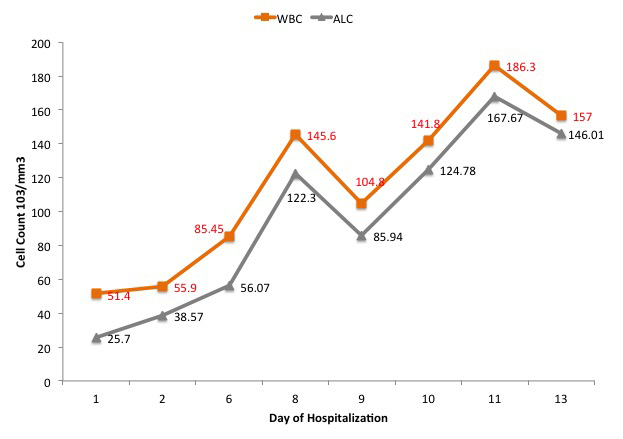
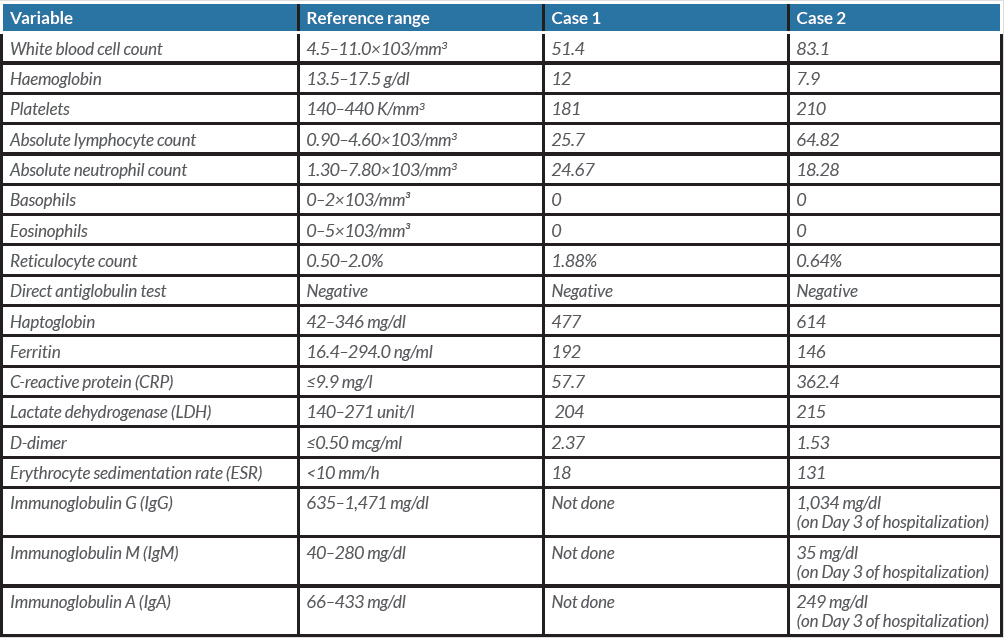
In the emergency department (ED), initial vital signs were significant for temperature 36.7°C, heart rate 106 beats/minute, respiratory rate 20 breaths/minute, blood pressure 145/62 mmHg, oxygen saturation of 96% on room air. The physical examination was unremarkable. Initial laboratory values are summarized in Table 1. The chest x-ray as seen in Fig. 1 showed clear lungs and no pleural effusions. The patient was hospitalized and placed on a nasal cannula and started on prednisone 20 mg BID on Day 1 of admission for 3 days followed by prednisone 20 mg orally daily for an additional 3 days. On Day 6 of hospitalization, the hospital course was complicated by atrial fibrillation with rapid ventricular response, treated with intravenous (IV) diltiazem. At that time, the patient was started on therapeutic Lovenox and also remdesivir for 5 days and was later switched to methylprednisolone 40 mg IV every 8 hours. The WBC count continued to trend upwards. The WBC trend during the entire hospitalization is shown in Fig. 2. A peripheral blood smear showed many mature lymphocytes with multiple smudge cells and no blasts. Peripheral blood flow cytometry showed: “Chronic lymphocytic leukaemia/small lymphocytic lymphoma: 91% of the total”. The patient’s respiratory status continued to improve, and she was hospitalized for 13 days and subsequently discharged to subacute rehabilitation in a stable condition.
Figure 1. Chest x-ray showing clear lung fields
Figure 2. Graph showing the white blood cell (WBC) count and absolute lymphocyte count (ALC) corresponding to the day of hospitalization
CASE DESCRIPTION 2
We report the case of a 79-year-old female, with a past medical history significant for CLL, hypertension and hypothyroidism, who presented to the ED complaining of fever, shortness of breath, dry cough and fatigue for 2 days. The symptoms were associated with intermittent fevers relieved by Tylenol, nasal congestion with discharge and dizziness. The patient was diagnosed with CLL in April 2013 and was under observation. The patient baseline counts were WBC 120 K. She denied any chest pain, nausea, vomiting or diarrhoea but reported positive exposure to COVID-19. The nasopharyngeal swab tested positive in the SARS-CoV-2 (rRT-PCR) assay.
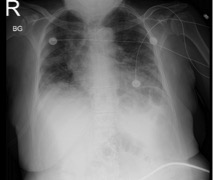
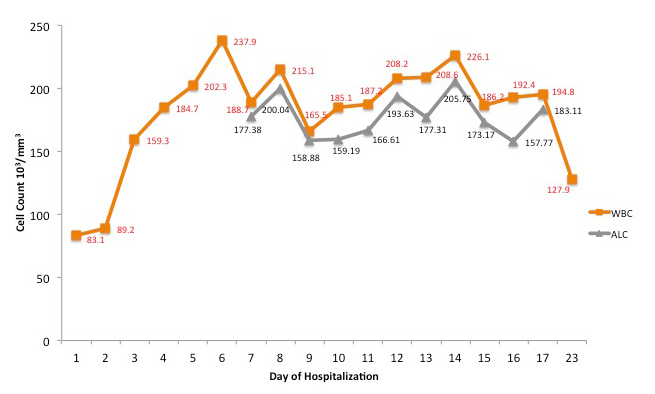
In the ED, initial vital signs were significant for temperature 36.9°C, heart rate 94 beats/minute, respiratory rate 24 breaths/minute, blood pressure 114/64 mmHg, SpO2 88% on room air. The physical examination was unremarkable. Relevant laboratory data are listed in Table 1. The chest x-ray as seen in Fig. 3 showed bilateral hazy infiltrates throughout both lung fields. The patient was initially placed on a nasal cannula and was started on azithromycin, ceftriaxone, dexamethasone and remdesivir. On Day 5 of hospitalization, the patient was noted to be in respiratory distress, and she was intubated and transferred to the intensive care unit (ICU) and managed for acute hypoxic respiratory failure and ARDS. The trend with respect to the WBC count and the absolute lymphocyte count is shown in Fig. 4. On Day 10 of hospitalization, the patient self-extubated. At that time, she was placed on a nonrebreather and oxygen saturation was approximately 96%. The patient's condition remained stable throughout and she was discharged to home on Day 24 of hospitalization.
Figure 3. Chest x-ray showing bilateral hazy infiltrates throughout both lung fields
Figure 4. Graph showing the white blood cell (WBC) count and absolute lymphocyte count (ALC) corresponding to the day of hospitalization
DISCUSSION
CLL is the most common leukaemia in adults in the Western world and is categorized by the presence of the proliferation of mature clonal B cells in the bloodstream, bone marrow, lymph nodes and spleen, which is confirmed by flow cytometry. A majority (80%) of patients are asymptomatic at the time of diagnosis [5].
The SARS-CoV-2 pandemic has led to profound morbidity and mortality around the globe. The outcome and disease course of COVID-19 in patients with haematological malignancies are not fully known. Mato et al. analyzed 198 patients with CLL and confirmed symptomatic COVID-19 in a multicentre study and concluded that irrespective of disease phase or status of treatment, CLL patients who were admitted are at high risk of death [6]. Lymphopenia has been associated with severe disease and is a poor prognostic factor in COVID-19-infected patients [7]. Vardanyan et al. reported the case of a 61-year-old patient with CLL who was diagnosed with COVID-19 and experienced a spontaneous partial resolution of CLL lymphocytosis for a short period [8]. However, contrary findings have also been reported; Paneesha et al. reported a case series of 4 CLL patients with COVID-19 with COVID-19-induced lymphocytosis [9]. Hyperleucocytosis is defined as a WBC count >100×109/l (100,000/μl) [10]. Leucostasis (also called symptomatic hyperleucocytosis) is a medical emergency and results from aggregation of leukaemic cells in the intravascular system, resulting in hypoxia and tissue injury [10]. Leucostasis is very rare in CLL and is more commonly seen in acute leukaemia [10].
Anaemia in COVID-19 could be due to multiple reasons; anaemia related to inflammation or bleeding due to anticoagulation [11]. Patients with CLL/SLL have an increased incidence of autoimmune haemolytic anaemia (AIHA). The direct antiglobulin (Coombs) test (DAT) may be positive in up to 35% of cases at some time during the course of the disease; overt AIHA occurs in approximately 10% of cases, usually later in the disease course [12]. There have been reports of AIHA associated with COVID-19 [13]. Clinicians should be hypervigilant with CLL patients with COVID-19 regarding haemolysis. Haemolytic work-up was negative in our case series.
In addition to advanced age seen in CLL, these patients are considered immunocompromised due to dysregulation of humoral immunity as well as cellular immunity, and are thus more prone to developing infections [14, 15]. This dysregulation is suggested by the presence of hypogammaglobulinaemia, and an impairment in the number and function of B and T cells [6]. Ye et al. reported a case of a 72-year-old female with a history of lymphocytosis of unknown aetiology who was admitted to hospital after developing COVID-19 as part of a household cluster. The patient was diagnosed with COVID-19 and newly diagnosed CLL. Compared to her family members, the patient suffered from severe pneumonia (PNA) with superimposed bacterial infection; however, she recovered and was discharged approximately 20 days after admission. The authors concluded that early intervention with antivirals and high-dose IV immunoglobulin (IVIG) appeared to be effective in this patient [16].
Langerbeins et al. reported a case of a 52-year-old male with multiple comorbidities and CLL who was admitted and managed in the ICU for COVID-19 PNA complicated by concomitant parainfluenza virus 4 infection. He was managed conservatively with high flow oxygen via a nasal cannula. The patient also received 30 g immunoglobulin G daily (IVIG) from Day 17 to Day 20. The patient was discharged on Day 28. The authors urged clinicians to conduct a complete examination of respiratory samples to rule out bacterial, viral and fungal superinfections in these patients and to administer immunoglobulins as per guidelines [17].
Alves Barbosa et al. reported a case of a patient with CLL and SARS-CoV-2 infection with a WBC count of 323×109 cells/l. The authors observed a discrepancy between persistently low measured PaO2 levels on ABG analysis and normal oxygen saturation. The authors drew attention to leucocytosis-induced pseudohypoxaemia due to increased consumption of dissolved oxygen in blood samples [18].
Finally, the decision to continue, or discontinue, antileukaemic therapy should be made on an individual basis. FDA-approved therapy for CLL includes the use of Bruton tyrosine kinase inhibitors (BTKis) such as ibrutinib; purine analogues such as fludarabine and pentostatin; the anti-CD20 monoclonal antibodies obinutuzumab, ofatumumab and rituximab and alkylating agents such as bendamustine, chlorambucil and cyclophosphamide [5]. The BTK pathway is crucial for the development of several pro-inflammatory cytokines; thus, inhibiting this process leads to decreased release of cytokines and results in an anti-inflammatory effect. Thus, inhibition of BTK may represent a potential mechanism to reduce the excessive inflammation/immune response produced by SARS-CoV-2 [19].
Several recent case series and reports support the possible protective effects of BTKis; however, there are still insufficient data to recommended continuing or discontinuing BTKis. In a recent study by Reda et al., the authors analyzed data from 2,902 patients with CLL, out of which 278 were on ibrutinib and 23 were noted to have confirmed SARS-CoV-2 infection. Among patients on ibrutinib, 4 patients were reported to have confirmed SARS-CoV-2 infection. One patient was managed as an outpatient while 3 patients developed ARDS with 2 requiring mechanical intubation. The patient with the highest burden of comorbidities died from COVID-19 complications. While the sample size was small, the authors speculated that perhaps the low prevalence (4/278) of COVID-19 amongst those on ibrutinib was due to its modulatory actions on the cell-mediated immune response and cytokine release [20]. Thibaud et al., in a cohort of 8 patients with CLL and COVID-19, observed that 2 patients who were continued on ibrutinib were characterized by a shorter hospitalization length, minimal oxygen requirements, and eventually, full recovery [19]. Lin et al. reported a case of a patient with CLL who was on ibrutinib and who then developed severe COVID-19 infection requiring mechanical ventilation. Ibrutinib was continued and the patient was extubated after 9 days and eventually discharged on room air, and the authors suggest that ibrutinib may have a protective effect by decreasing inflammation and preventing progression to ARDS [21].
CONCLUSION
While some studies concluded that the overall prevalence of COVID-19 in patients with CLL is not substantially higher than the average population, the fact remains that these patients are at increased risk of acquiring infections due to the inherent role of an impaired immune response, and thus, care needs to be taken by both clinicians and patients to prevent infection.
Therapy in individuals with CLL should be personalized, based on the overall medical history and clinical status. In conclusion, we report 2 cases of COVID-19-induced hyperleucocytosis (WBC count >100,000/μl) in CLL patients and further studies, perhaps randomized, are essential to examine the outcome of COVID-19 in patients with CLL and to clarify the role of BTKi therapy at the time of the pandemic.