ABSTRACT
Hypereosinophilic syndrome (HES) is a heterogenous group of diseases characterized by abnormal accumulation of eosinophils in the blood or peripheral tissues. It can affect all organs and therefore clinical manifestations are highly variable. We describe the case of a 38-year-old man admitted for febrile polyserositis. He developed cardiac tamponade requiring pericardiocentesis complicated by left ventricle perforation which was successfully repaired. He presented mild peripheral eosinophilia. Bronchoalveolar lavage evidenced eosinophilic alveolitis, and pleural and pericardium histopathology revealed the presence of abundant eosinophils. All other causes of tissue eosinophilia were excluded and the diagnosis of idiopathic HES was made. The patient was started on glucocorticoids with resolution of symptoms. This case report describes a rare but potentially fatal presentation of HES and demonstrates the difficulty and delay in diagnosis when peripheral hypereosinophilia is absent.
LEARNING POINTS
- Hypereosinophilic syndrome (HES) is characterized by abnormal accumulation of eosinophils in the blood or peripheral tissues.
- The clinical manifestations of HES are highly variable.
- It may be difficult to diagnose HES when peripheral hypereosinophilia is absent.
KEYWORDS
Hypereosinophilic syndrome, polyserositis, eosinophilic alveolitis, eosinophilic pleurisy, eosinophilic pericarditis
INTRODUCTION
Hypereosinophilic syndrome (HES) is a heterogenous group of rare diseases characterized by abnormal accumulation of eosinophils in the blood or peripheral tissues [1]. Any system can be affected, but the most common are the cutaneous, cardiovascular, pulmonary and haematological systems [2]. Tissue damage is more likely to occur when the absolute eosinophil count (AEC) exceeds 1500/µl, but peripheral blood eosinophilia does not accurately predict the risk of organ damage. Clinical manifestations are highly variable, ranging from asymptomatic eosinophilia to end-organ failure, developing insidiously or acutely with rapid progression [2, 3]. Glucocorticoids are used as a first-line agent followed by the introduction of a steroid-sparing agent if needed [3]. Imatinib mesylate and other immunomodulatory agents have shown promising results [2].
CASE DESCRIPTION
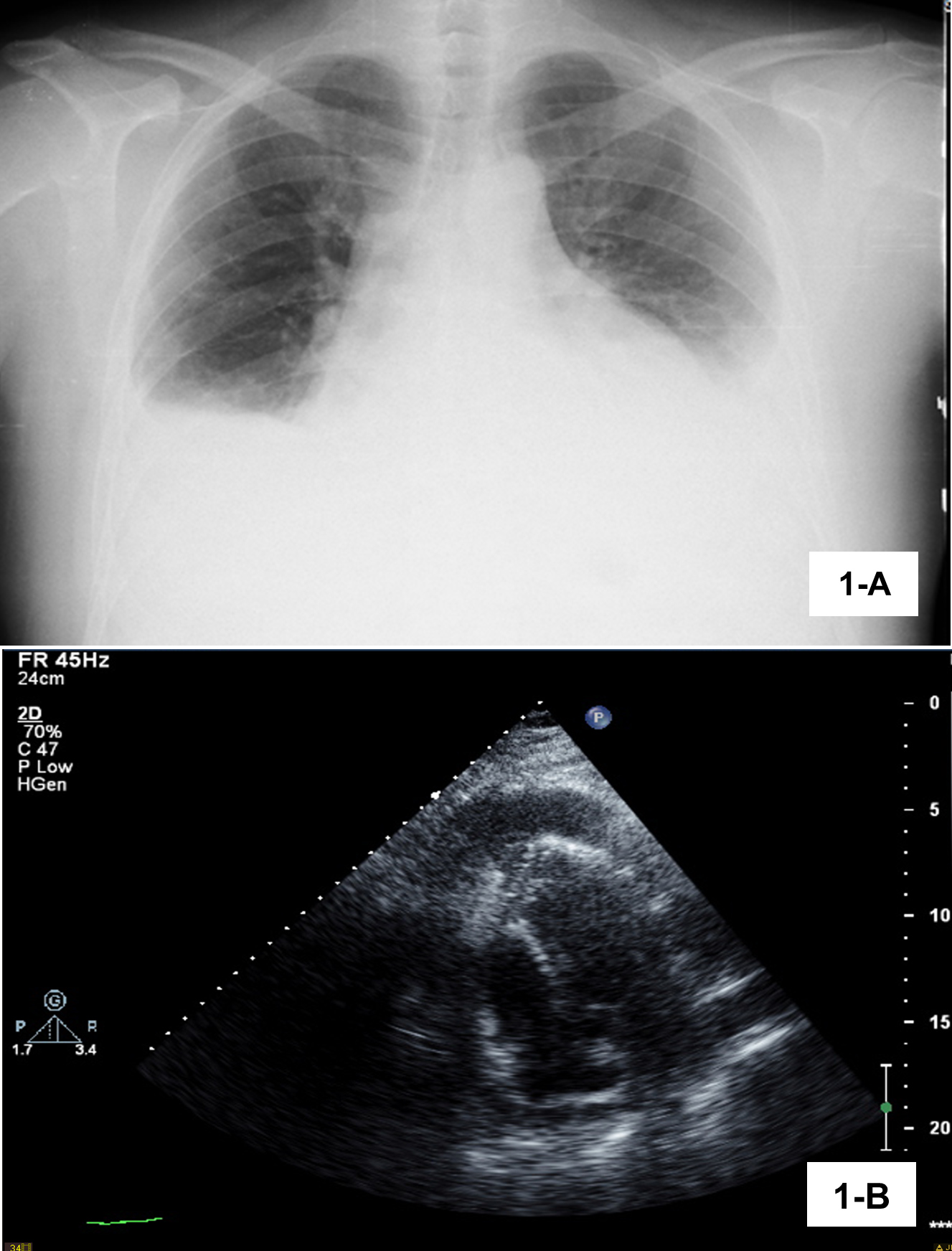
A 38-year-old male chef was admitted to the emergency department with dyspnoea on moderate exertion and a 1-month history of pleuritic chest pain with progressive worsening. He had experienced evening fever since the previous week, dry cough, asthenia, and an episode of paroxysmal nocturnal dyspnoea the night before admission. He had no previous diseases and was not taking regular medication, but did have contact with a patient being treated for active pulmonary tuberculosis 3 years previously. On physical examination, he was febrile, tachycardic, tachypnoeic and had respiratory insufficiency requiring supplemental oxygen. Pulmonary auscultation demonstrated absent breath sounds in both lung bases. The remaining physical examination was unremarkable, including the skin and testis. Laboratory findings revealed eosinophil count 230/µl (<700), C-reactive protein 17 mg/dl (<0.5), sedimentation rate 85 mm/hr (<15) and N-terminal proB-type natriuretic peptide 55 pg/ml (<125). There was bilateral pleural effusion on chest radiography (Fig. 1A) and computed tomography (CT) angiography of the thorax showed pleural and pericardial effusion. There was vestigial intraperitoneal free fluid on abdominal ultrasound. The electrocardiogram was unremarkable. The patient was hospitalized for febrile polyserositis. The transthoracic echocardiogram revealed moderate pericardial effusion with signs of haemodynamic compromise (Fig. 1B) resulting in cardiac tamponade. The patient required pericardiocentesis which was complicated by left ventricle perforation, followed by cardiac arrest and successful emergent surgical correction.
Figure 1 (A) Chest x-ray on admission showing bilateral pleural effusion. (B) Transthoracic echocardiogram showing moderate pericardial effusion with signs of haemodynamic compromise.
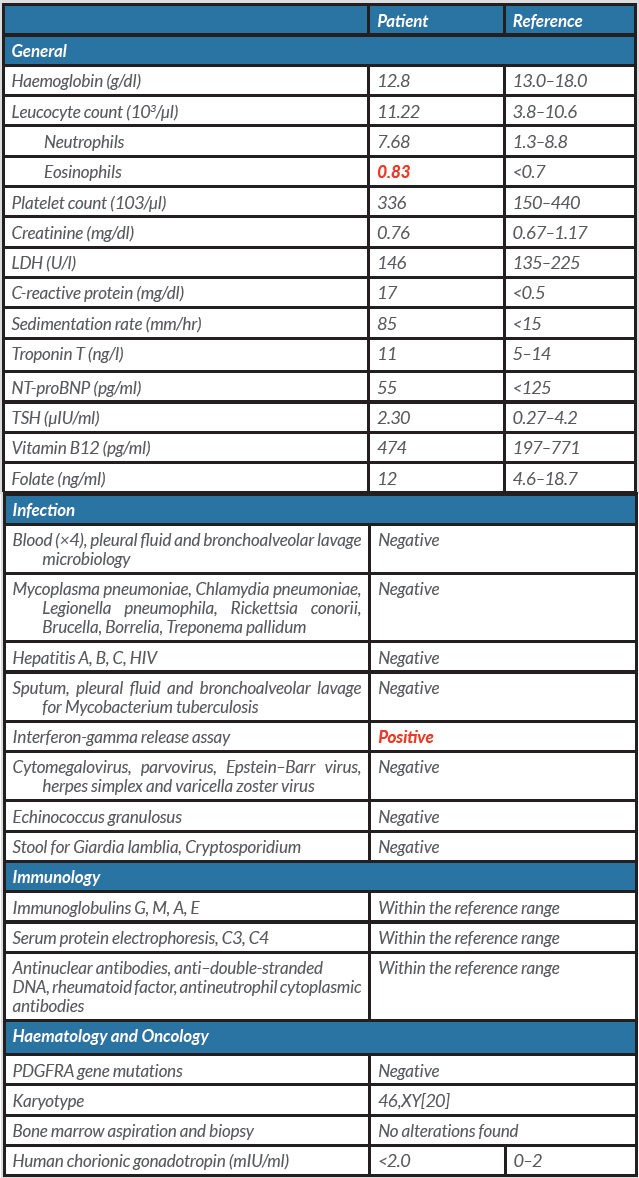
After a period of recovery in the surgical ward, the patient was readmitted to internal medicine care for further study. We were able to exclude infectious, autoimmune, endocrine and haematological aetiologies (Table 1). The patient tested positive on an interferon-gamma release assay (IGRA). CT scanning of the thorax, abdomen and pelvis as well as a positron emission tomography scan revealed no additional findings.
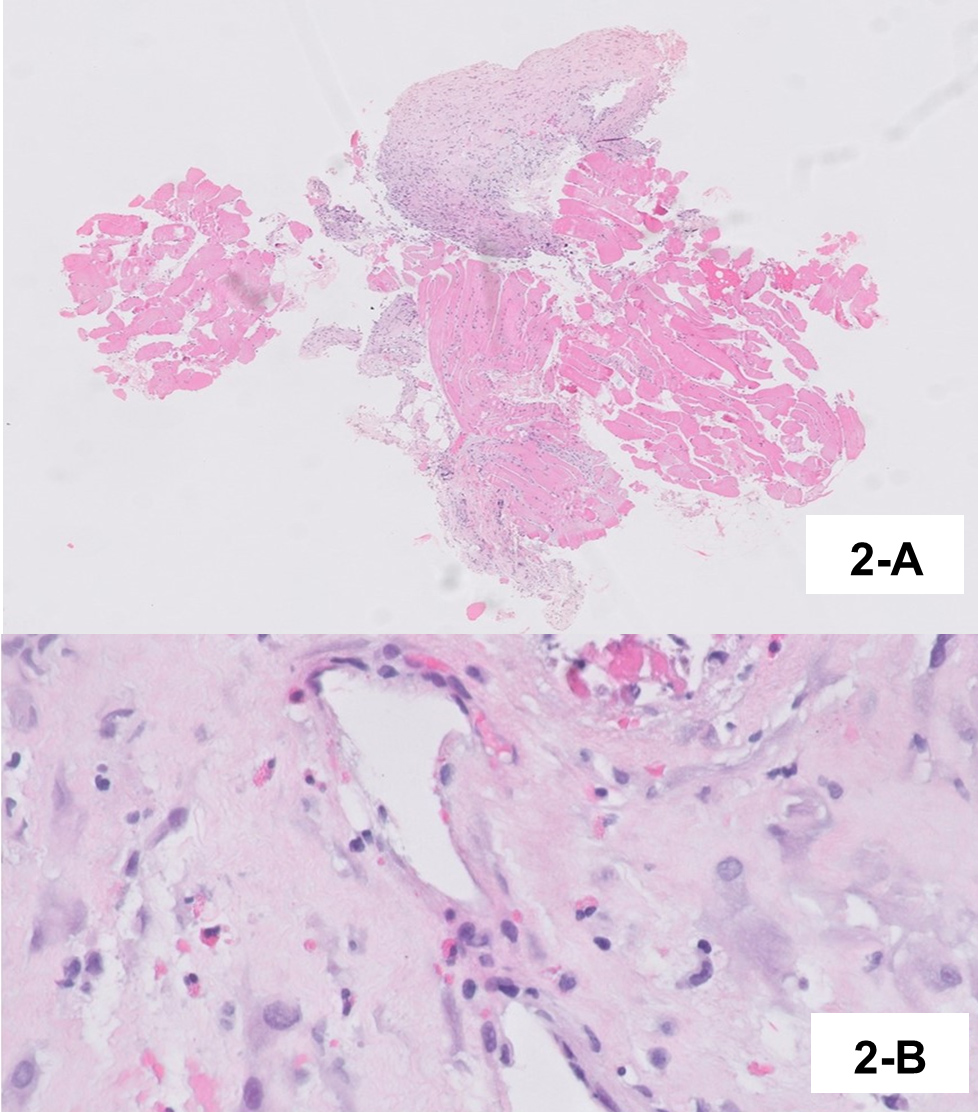
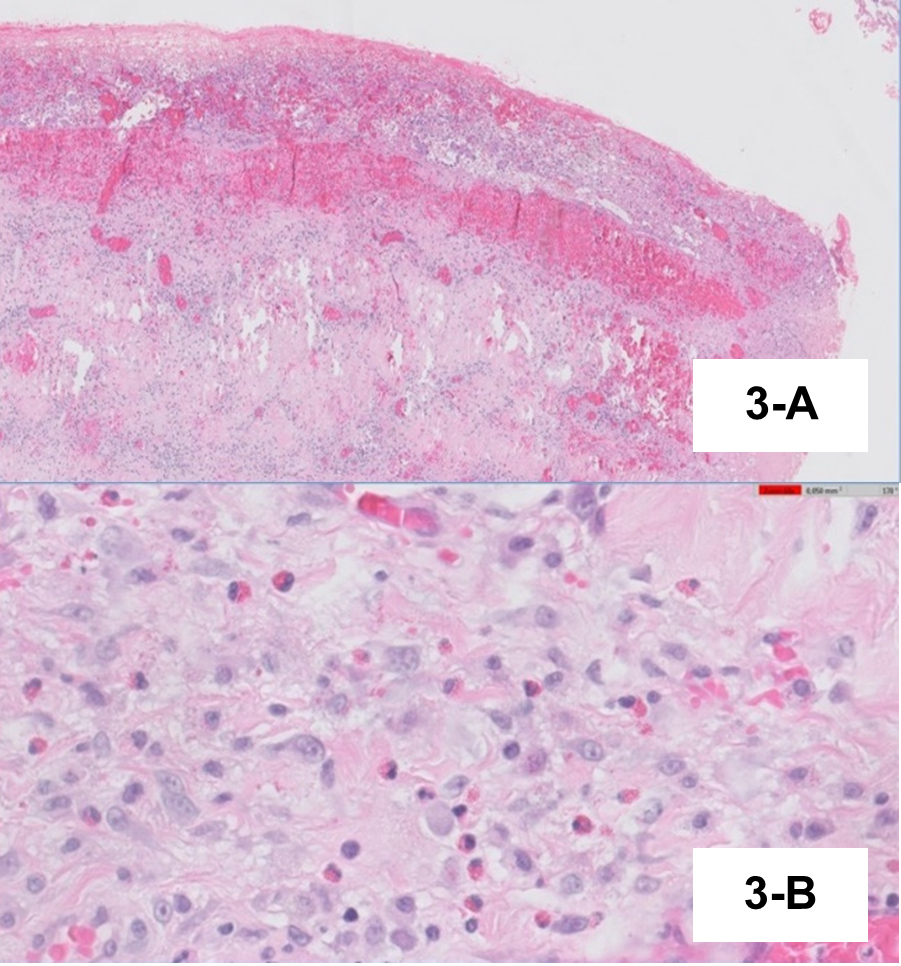
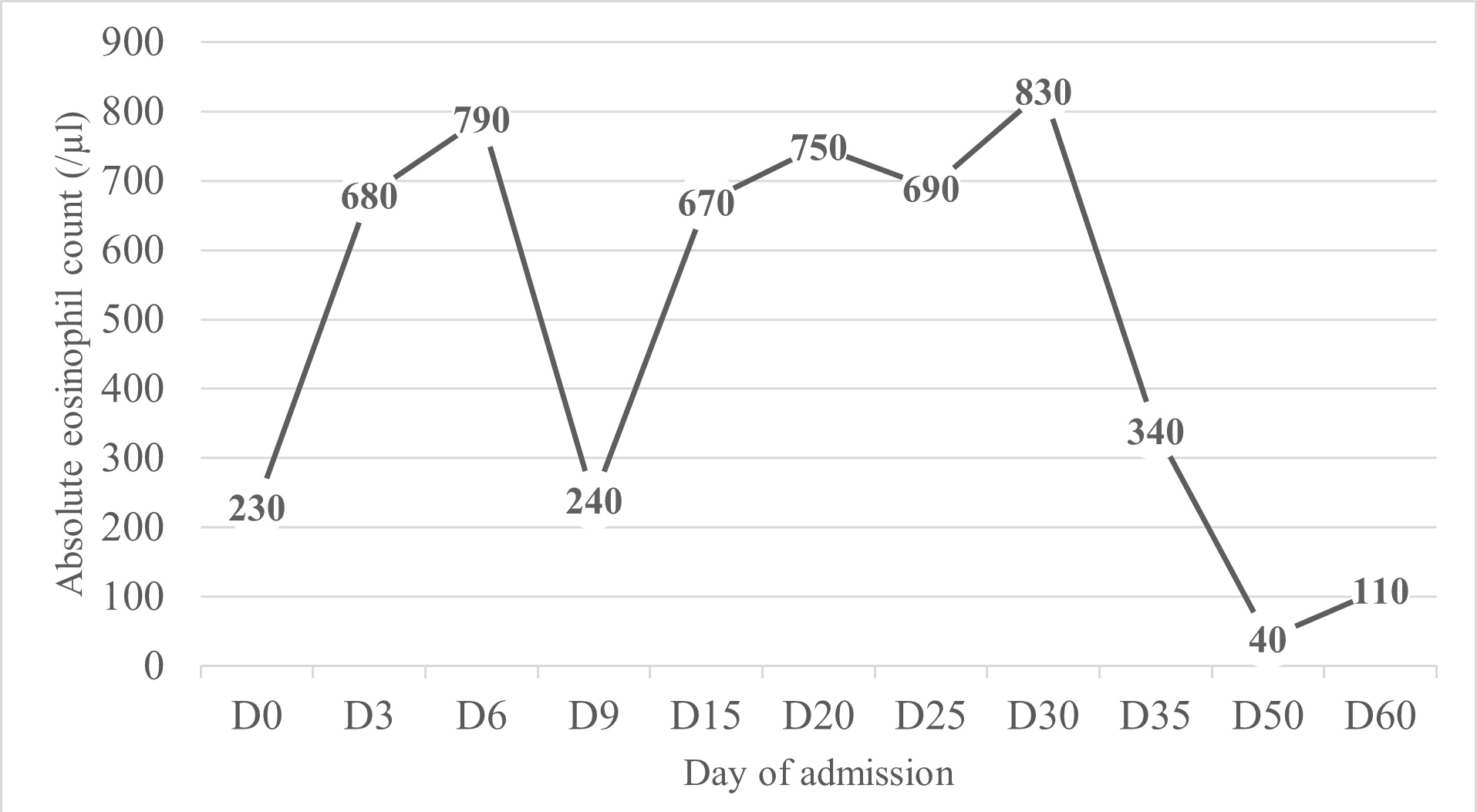
Pleural fluid examination showed characteristics of exudate with mononuclear predominance (1043/µl leucocytes with 55.7% mononuclear cells). Bronchoalveolar lavage evidenced eosinophilic alveolitis (6.2% eosinophils) with negative cultures. Histopathological examination of pleura was consistent with subacute pleurisy with eosinophils (Fig. 2), and pericardium histopathology revealed subacute fibrinous pericarditis with abundant eosinophils (Fig. 3). The peak of AEC in peripheral blood was 830 cells/µl (Fig. 4). We did not find any genetic rearrangements of the PDGFRA or PDGFRB genes on peripheral blood. Based on these findings, the diagnosis of idiopathic HES was made and the patient was started on 1 mg/kg/day of prednisolone. Tuberculosis prophylaxis with isoniazid was also instituted. He showed clinical improvement within the next few days and was discharged home. The dose of corticosteroid was gradually tapered and discontinued after 6 months. Five years later, the patient remains on regular follow-up with no evidence of disease recurrence.
Figure 2 (A) Skeletal muscle of the chest wall and pleura thickened by fibrosis and inflammatory infiltrate. (B) Thickened pleura, with inflammatory infiltrate rich in eosinophils
Figure 3 (A) Thickened pericardium, with fibrinoleucocyte exudate and subacute haemorrage and underlying fibrosis. (B) Eosinophilic infiltrate
Figure 4. Absolute eosinophil count during hospital admission
Table 1. Laboratory findings - Values in red are out of range
DISCUSSION
Cardiac involvement in HES is common [4], but pericardial involvement is estimated to be less than 10% [5]. There are several case reports on HES presenting as cardiac tamponade [4–9], but to our knowledge this is the first case without peripheral hypereosinophilia. Our patient presented with only mild peripheral eosinophilia which led to a delay in diagnosis. Only when histopathology reports showed tissue damage due to presence of eosinophils did the diagnosis became apparent. In the setting of HES, patients usually present with peripheral blood hypereosinophilia and eosinophilia-related target organ damage [3]. However, it is worth mentioning that the diagnostic criteria include tissue infiltration that is extensive in the subjective opinion of a pathologist [1]. The diagnosis of idiopathic HES was established as all other known causes of eosinophil dysfunction were excluded. The aim of therapy in HES is to reduce the eosinophilic granulocyte count in tissues, and glucocorticoids are generally recommended as first-line therapy [4, 7]. Our patient responded well to prednisolone with iatrogenic diabetes during treatment that was well controlled with metformin and sitagliptin. However, if prednisolone is not suitable, many other regimens are available, including steroid-sparing agents, a tyrosine-kinase inhibitor (imatinib) or immunomodulatory agents [2, 3]. Because of profound immunosuppression during treatment, it is important that infectious diseases are excluded. Our patient had previous contact with a patient being treated for active pulmonary tuberculosis a few years earlier and tested positive for IGRA. Therefore, it was mandatory to exclude active infection and start treatment for latent tuberculosis.
CONCLUSION
The authors consider that this case is unique because of the severity of organ dysfunction (eosinophilic alveolitis, pleurisy and pericarditis) in the absence of hypereosinophilia. Clinical expertise and attention are needed in the diagnosis of rare diseases, especially with atypical presentations, to ensure the best outcomes.