ABSTRACT
We present the case of a 33-year-old woman who was diagnosed with disseminated Kaposi's sarcoma and HIV infection. The patient improved on highly active antiretroviral therapy (HAART), however, 9 days into treatment, she became febrile and dyspnoeic and developed tender cervical and axillary lymphadenopathy. Despite treatment for suspected sepsis and immune reconstitution, she died in intensive care. Lymph node biopsies revealed coexistent Castleman's disease and Kaposi's sarcoma. Initiation of HAART can be rarely associated with unmasking and rapid progression of Castleman's disease, a phenomenon called immune reconstitution. Urgent investigation and treatment with agents such as steroids and cytotoxic drugs can be life-saving.
LEARNING POINTS
- Multicentric Castleman's disease tends to present with lymphadenopathy and various non-specific symptoms, especially in the context of HIV infection.
- HAART can rarely unmask symptoms of multicentric Castleman's disease, a phenomenon called immune reconstitution inflammatory syndrome.
- Since multicentric Castleman's disease can be rapidly fatal in the context of HIV infection, lymph node biopsy should be undertaken early so that treatment can be started urgently.
KEYWORDS
Kaposi's sarcoma; multicentric Castleman's disease; immune reconstitution inflammatory syndrome.
INTRODUCTION
Lymphadenopathy in the context of Kaposi's sarcoma and HIV infection can be due to numerous causes, including metastatic Kaposi's sarcoma and opportunistic infections. However, it should alert the clinician to the possibility of coexistent Castleman's disease, a rare lymphoproliferative disorder which shares the same viral aetiology as Kaposi's sarcoma[1]. We report a case of an HIV-positive patient with Kaposi's sarcoma who developed rapidly progressive Castleman's disease shortly after initiation of highly active antiretroviral therapy (HAART).
CASE PRESENTATION
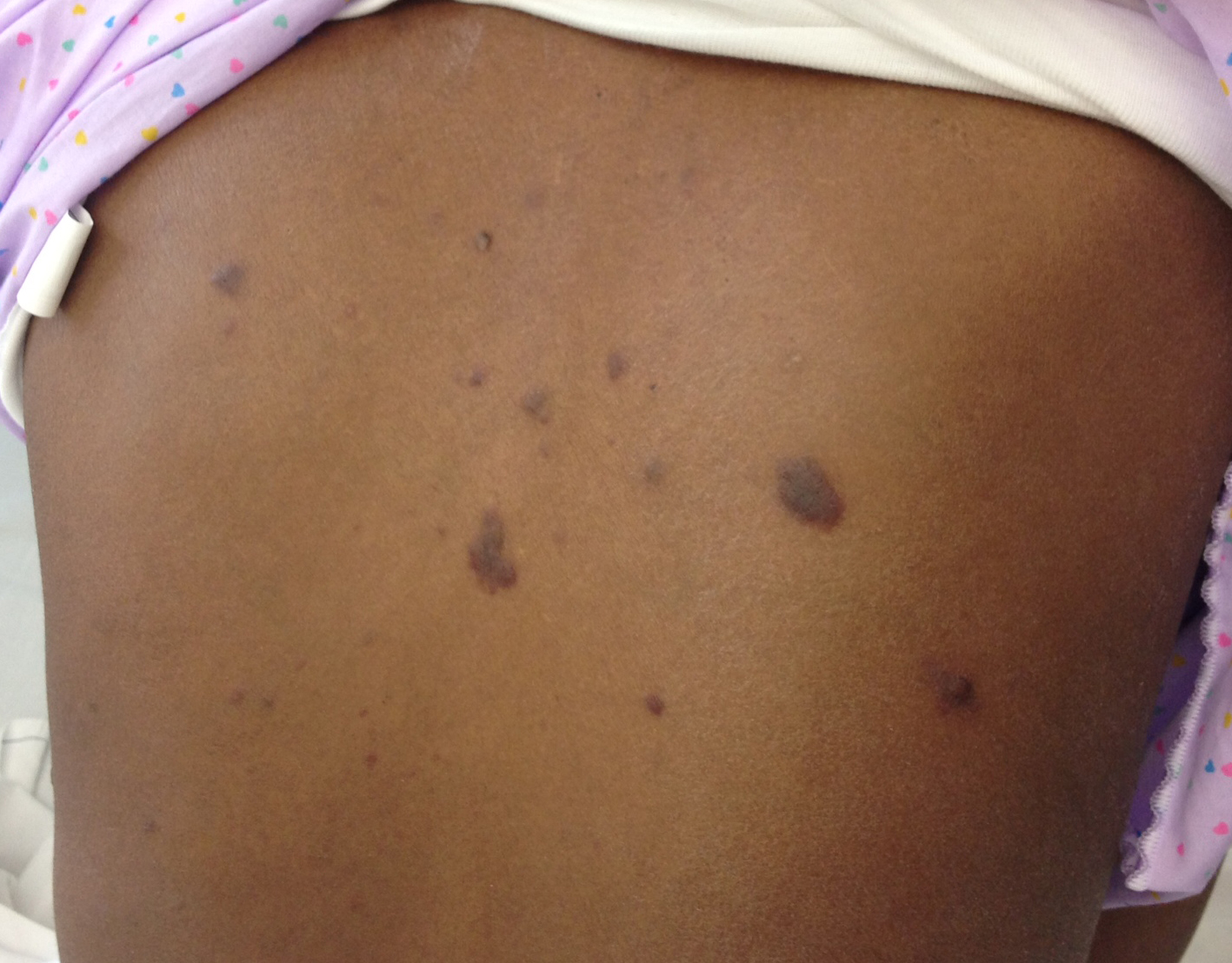
A 33-year-old female Somali refugee presented to hospital with a 3-month history of fever, productive cough, haemoptysis, night sweats, weight loss and a skin eruption. On examination, there were several violaceous nodules and plaques up to 2.5 cm in diameter, suggestive of Kaposi's sarcoma, on the trunk (Fig. 1), upper limbs and oral mucosa as well as bilateral diffuse crepitations in the chest. There was no clinical evidence of lymphadenopathy at presentation.
Figure 1: Violaceous nodules and plaques suggestive of Kaposi's sarcoma on the back of the patient.
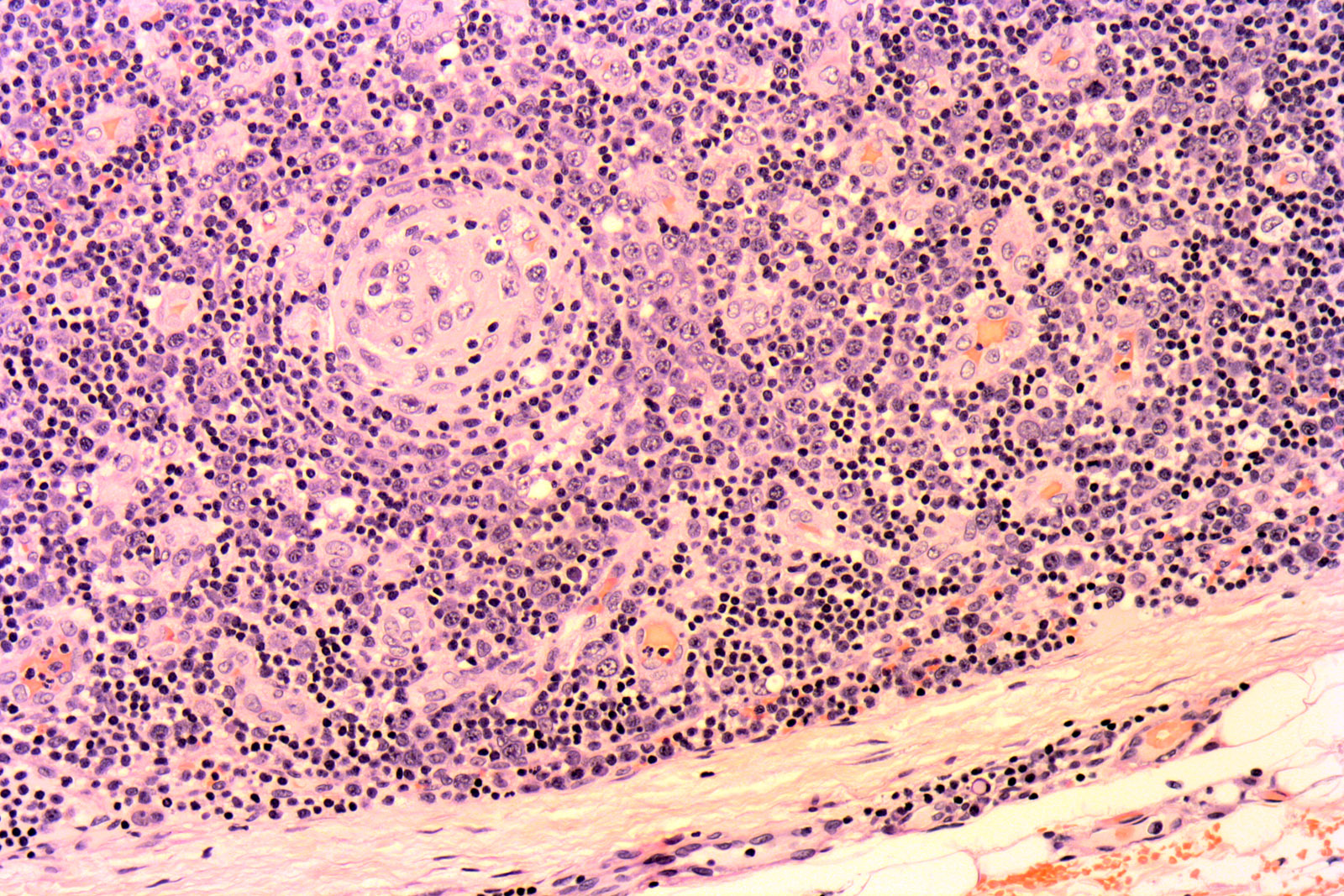
Figure 2: H&E ×100: The photomicrograph shows the follicular hyperplasia, narrow mantle zone and sheets of plasma cells from a lymph node affected by Castleman's disease.
The patient was admitted to hospital where an HIV antibody test, serial sputa test for acid-fast bacilli and blood culture tests, including cultures for mycobacteria, were carried out. A chest X-ray revealed diffuse reticulonodular shadowing. A computed tomography (CT) scan of the thorax was suggestive of disseminated pulmonary Kaposi's sarcoma with axillary and retroperitoneal lymphadenopathy. Serial sputa were negative for acid-fast bacilli. Empiric treatment for Pneumocystis jirovecii pneumonia was started with a combination of oral co-trimoxazole and prednisolone. On Day 6, when it was confirmed that the patient was HIV positive, with a CD4 count of 247/mm3, HAART was initiated using a combination of lopinavir/ritonavir and lamivudine/zidovudine. On treatment, the patient's fever and respiratory symptoms improved. Clinically, some regression of the cutaneous nodules and plaques was noted. However, on Day 14, the patient again developed high fever, night sweats, lateral neck pain, nausea and vomiting. She became progressively pancytopenic and hypoalbuminaemic. Her erythrocyte sedimentation rate (ESR) rose to 95 mm/h and C-reactive protein (CRP) to 144 mg/dl from previously normal levels prior to initiation of HAART. On examination, multiple tender, firm cervical and axillary lymph nodes were palpable. Skin and lymph node biopsies were organized and a chest X-ray showed that infiltrates were now much more diffuse. The patient was treated empirically with intravenous broad-spectrum antibiotics and intravenous hydrocortisone for possible immune reconstitution inflammatory syndrome (IRIS) and sepsis. However, she continued to deteriorate and on Day 20 was admitted to the Intensive Care Unit with respiratory failure and died within a few hours.
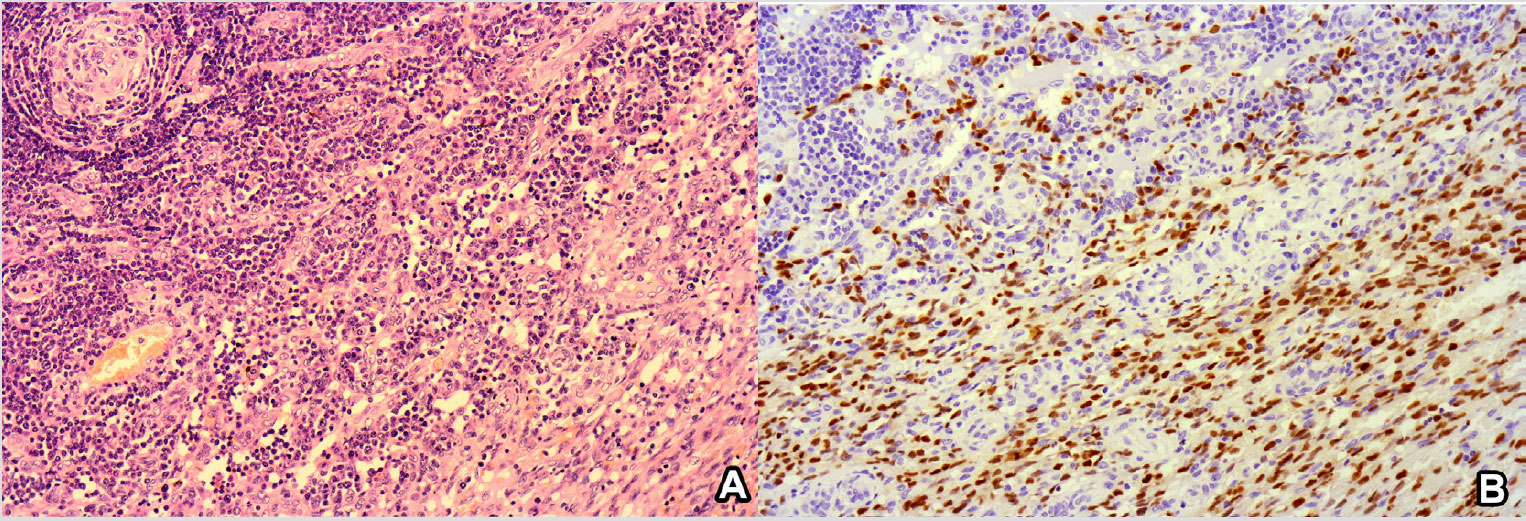
Blood cultures did not reveal microbial growth. The skin biopsy demonstrated a spindle cell proliferation arranged in fascicles forming cleft-like spaces that contained erythrocytes infiltrating the dermis. Haemosiderin deposition was also observed. The lymph nodes showed a similar spindle cell proliferation, and follicular hyperplasia with a narrow mantle zone surrounded by sheets of plasma cells. The germinal centres contained dendritic and endothelial cells and appeared partly hyalinized (Fig. 2). Immunohistochemistry revealed diffuse expression of CD31 and HHV8 by the spindle cells in the skin and lymph nodes. HHV8 was also expressed in the abnormal germinal centres. These findings were compatible with Kaposi's sarcoma affecting the skin and lymph nodes; with the multicentric variant of the plasma cell type of Castleman's disease within lymph nodes (Fig. 3. The fact that Castleman's disease progressed so rapidly and presented after commencing HAART is compatible with IRIS.
Figure 3A: H&E ×100: The photomicrograph shows a germinal centre with a partly hyalinized core and a narrow mantle zone surrounded by sheets of plasma cells in the upper left corner (Castleman's disease) and a spindle cell infiltrate with cleft-like spaces containing erythrocytes (Kaposi's sarcoma) in the lower right corner.
Figure 3B: HHV8 ×100: The photomicrograph shows strong nuclear positivity by both the spindle cells and the lymphoid cells surrounding the abnormal germinal centre.
DISCUSSION
Kaposi's sarcoma is a vascular spindle cell tumour that is an AIDS-defining illness. The association between Kaposi's sarcoma and human herpesvirus-8 was first reported in 1994 and most of the spindle and endothelial cells within Kaposi's sarcoma are infected with HHV-8[1].
Castleman's disease is a rare lymphoproliferative disorder in which HHV-8 infection also plays an important role in the aetiology[2]. The unicentric form of the disease is often discovered incidentally as a mediastinal mass on imaging and accounts for around 90% of cases[2]. The multicentric variant tends to feature constitutional symptoms including high fevers, night sweats, fatigue, anorexia and weight loss.
Lymphadenopathy is nearly universal and hepatosplenomegaly is present in around half of the cases[2]. Common laboratory abnormalities include anaemia, thrombocytopenia, elevated ESR and CRP, deranged liver profile, hypoalbuminaemia and renal dysfunction[2]. The disease tends to follow an aggressive course and flares can be fatal[2]. In addition, since the symptoms and laboratory abnormalities are very non-specific, the condition is often diagnosed late or missed.
The coexistence of Castleman's disease and Kaposi's sarcoma has been reported previously since both are associated with HHV-8[2]. What was unusual in our patient is that the symptoms of Castleman's disease became evident and progressed so rapidly within days of initiation of HAART. This unmasking of an occult disease after initiation of antiretroviral therapy is referred to as immune reconstitution inflammatory syndrome (IRIS). While it is recognised that Kaposi's sarcoma tends to improve with HAART, data regarding the effect of HAART on multicentric Castleman's disease is conflicting[2,3]. While some report successful treatment of the condition with HAART alone, others claim that multicentric Castleman's disease does not regress with HAART alone[2–4].
Several treatment options have been described for multicentric Castleman's disease, however because of the variety of clinical contexts and the rarity of the disease, there is no standard treatment yet. Rituximab is promising, however, it can lead to progression of Kaposi's sarcoma[2]. Chemotherapeutic agents such as doxorubicin, etoposide and vinblastine may be considered when multicentric Castleman's disease coexists with Kaposi's sarcoma[2,5].
Multicentric Castleman's disease is a rapidly progressive condition and the key to optimum management lies in early recognition of its clinical features and relevant investigations to confirm the diagnosis. Patients started on HAART should be monitored for features of this disease, which may require management as a medical emergency[4].